Thursday Feb. 25, 2010
click here to download today's notes in a more
printer friendly format
The music today was from Duffy ("Rockferry" and
"Mercy" and "Distant Dreamer").
A friend just recently told me about her. She had at least one
song in the recent movie "An Education."
The Experiment #2 reports are due next Tuesday. You can
still return
the
materials and pick up the supplementary information sheet this
week or early next week.
You'll need to come by my office in PAS 588
between about 9:30 and 5 pm. You'll find a box just inside the
door
and copies of the information sheet nearby.
The revised Expt. #1 reports are due Thursday next week (please
return the original report).
The first of the 1S1P Bonus Assignments
is due Tuesday next week.
There was a 2-question in-class optional assignment today.
You'll
find both questions at the end of today's notes. If you weren't
in
class and are reading these notes you can turn in answers to the
questions at the start of class next Tuesday and receive at least
partial
credit.
We first reviewed some material (temperature, heat, and temperature
scales) that was stuck onto the end of the online
notes
from last Tuesday. Material that we weren't able to
cover in class because of the fire alarm.
Conduction
is the first of four energy transport processes
that we
will cover (the least important transport process in the
atmosphere). The figure below illustrates this process. A
hot object is stuck in the middle of some air.
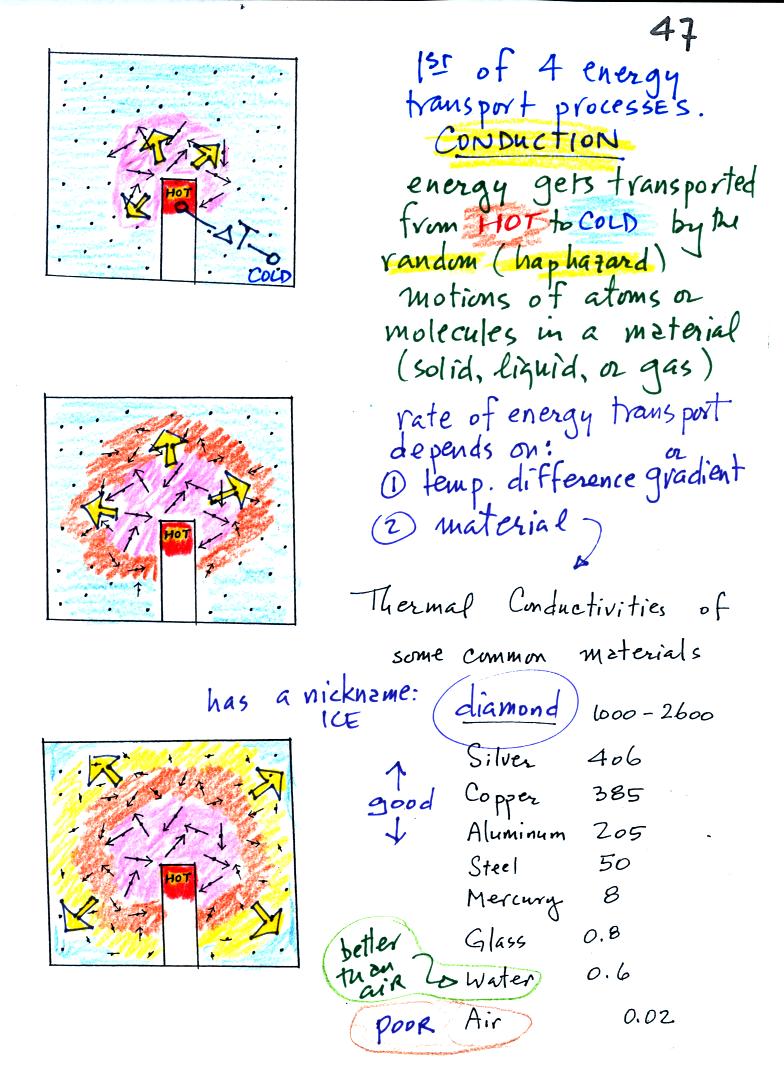
In the top picture some of the
atoms or molecules near the
hot object have collided with the object and picked up energy from the
object. This is reflected by the increased speed
of motion or increased kinetic energy of these molecules or
atoms (these guys are colored pink).
In the middle picture the
initial bunch of
energetic molecules have
collided with some of their neighbors and shared energy with
them (these are orange). The neighbor molecules have gained
energy though they don't
have as much energy as the molecules next to the hot object.
In
the third picture molecules further out have now (the yellow ones)
gained
some energy. The random motions and collisions
between molecules
is carrying energy from the hot object out into the colder material.
Conduction transports energy from hot to cold. The rate of
energy transport depends first on the material (air in the example
above). Thermal
conductivities of some common materials are listed. Air is a very
poor conductor of energy. Air is generally regarded as an
insulator. Water is a little bit better conductor. Metals
are generally very good conductors (cooking pans are often made of
stainless steel but have aluminum or copper bottoms to evenly spread
out heat when placed on a stove). Diamond has a very high
thermal conductivity. Diamonds are sometimes called "ice."
They feel cold when you touch them. The cold feeling is due to
the fact that they conduct energy very quickly away from your warm
fingers when you touch them.
The rate of energy transport also depends on
temperature
difference. If the object in the picture had been warm rather
than hot, less energy would flow or energy would flow at a slower into
the surrounding material.
Transport of energy by conduction is similar to the
transport of a strong smell throughout a classroom by diffusion.
Small eddies of wind in the classroom blow in random directions and
move smells throughout the room.. For our demonstration we used
curry powder.
With time the smell seemed to have
spread throughout about half the room. A second pile of curry
powder was placed in the back of the room so that everyone would get a
chance to smell it.
Because
air has such a low thermal conductivity it is often used as an
insulator. It is important, however, to keep the air trapped in
small pockets or small volumes so that it isn't able to move and
transport energy by convection (we'll look at convection
shortly). Here are some examples of
insulators that use air:
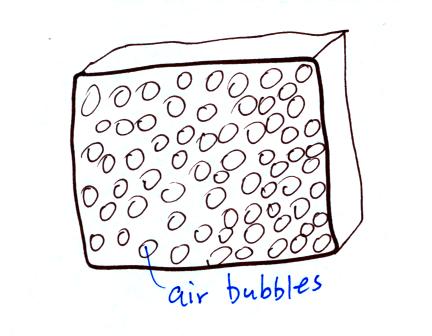
Foam is filled with lots of small air bubbles (it is
important that the bubbles be small otherwise convective air currents
become establised; we'll see that convection is a more efficient energy
transport process than conduction)
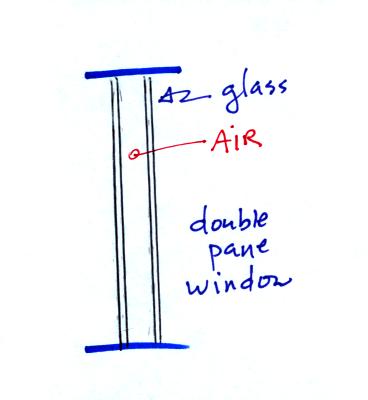
Thin insulating layer of air in a double
pane window
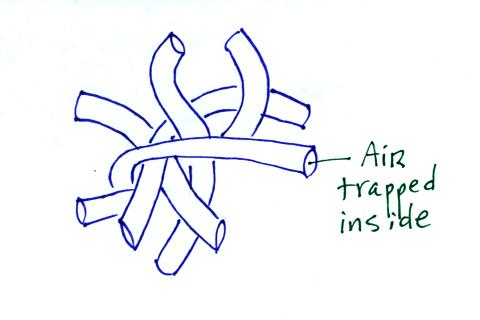
Hollow fibers (Hollofil) filled with air used in
sleeping
bags and
winter coats. Goose down works in a similar way.
Convection
was the next energy transport process we had a look at. Rather
than moving about randomly, the atoms or molecules move as a
group. Convection works in liquids and gases but not
solids.
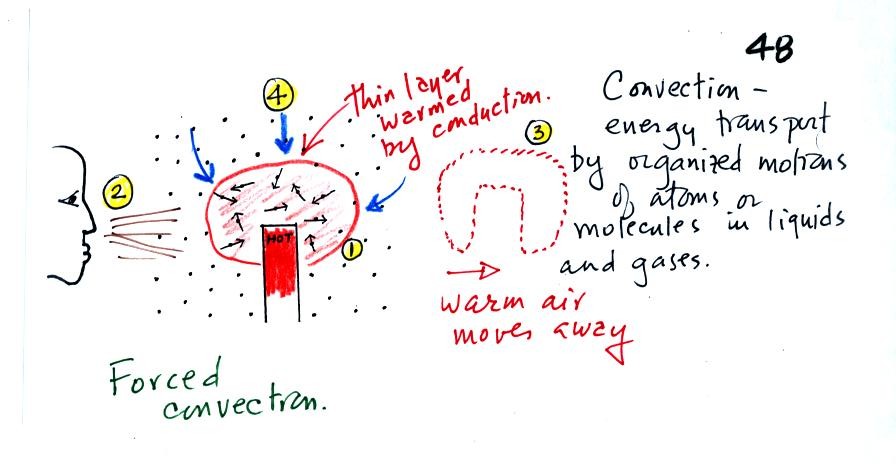
At Point 1 in the picture above a
thin layer of air
surrounding a hot object has
been
heated by conduction. Then at Point 2 a person (yes, that is a drawing
of a
person's head) is blowing the blob of warm air
off to the right. The warm air molecules are moving away at Point
3 from the
hot object together as a group (that's the organized part of the
motion). At Point 4 cooler air moves in and surrounds the hot
object and the whole process can repeat itself.
This is forced
convection. If you have a hot object in your hand you could just
hold onto it and let it cool by conduction. That might take a
while because air is a poor conductor. Or you could blow on the
hot object and force it to cool more quickly. I had wanted to put
a
small fan behind the curry powder to help spread the
smell further out into the classroom, but I forgot to bring the fan.
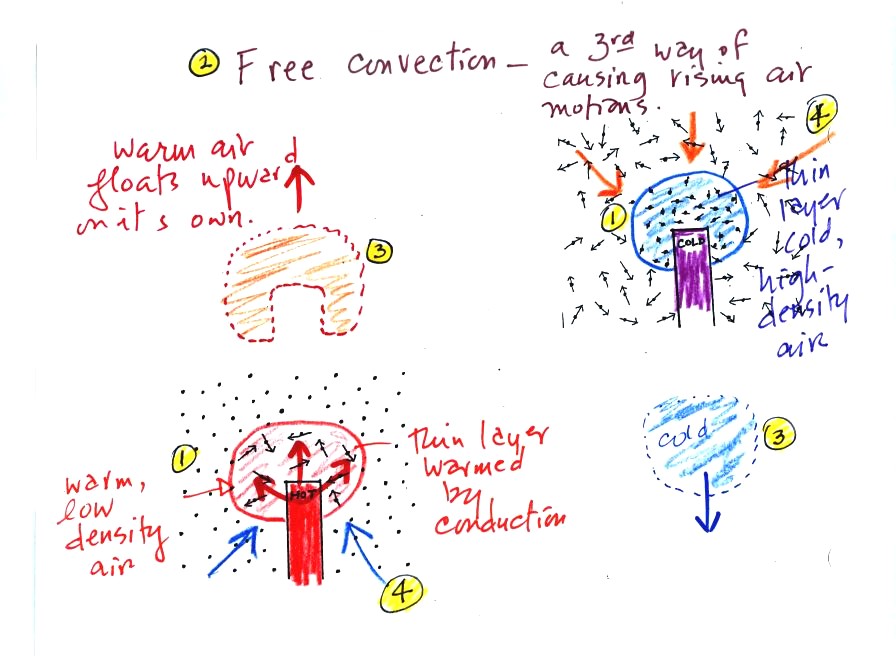
A thin layer of air at Point 1 in
the figure above (lower
left) is
heated by conduction. Then because hot air is also
low density air, it actually isn't necessary to blow on the hot object,
the
warm air will rise by itself (Point 3). Energy is being
transported away
from the hot object into the cooler surrounding air. This is
called free convection and
represents another way of causing rising air motions in the
atmosphere. Cooler air moves in to take the place of
the rising air at Point 4 and the cycle repeats itself.
The example at upper right is also
free convection. Room temperature air in contact with a cold
object loses energy and becomes cold high density air. The
sinking
air motions that would be found around a cold object have the effect of
transporting energy from the room temperature surroundings to the
colder object.
In both examples of free convection, energy is being transported
from
hot toward cold.
Now some
practical applications of what we have learned about conductive and
convective energy transport. Energy transport really does show up
in a lot more everyday real life situations than you might expect.
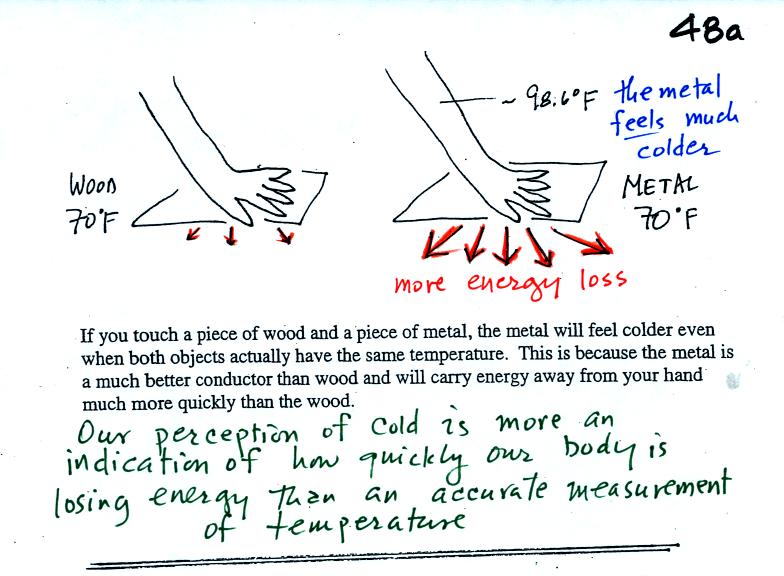
Note first of all there is a temperature difference between
your hand and a 70 F object. Energy will flow from your warm
hand to the colder object. Metals are better conductors than
wood. If you touch a
piece of
70 F metal it will feel much colder than a piece of 70 F wood, even
though they both have the same temperature. A
piece
of 70 F diamond would feel even colder because it is an even better
conductor
than metal.
Something that feels cold may not be as
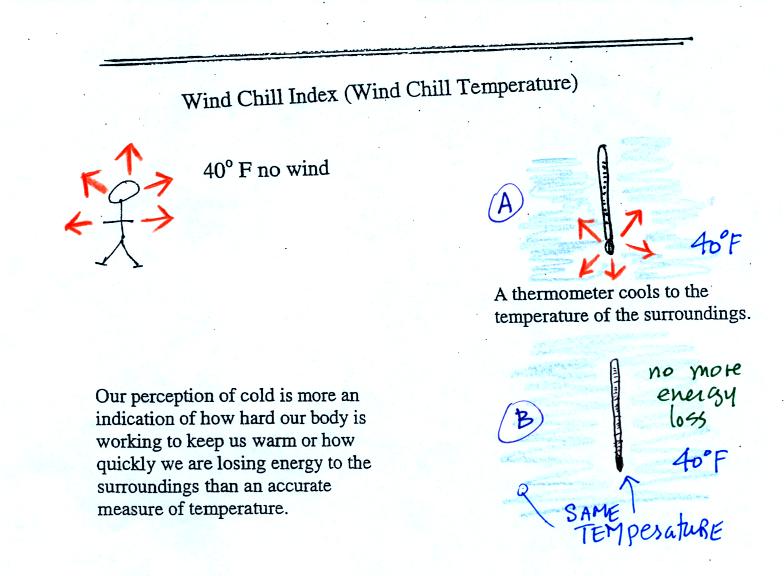
cold as it seems. Our perception of cold is more an
indication of how
quickly our hand is losing energy than a reliable measurement of
temperature.
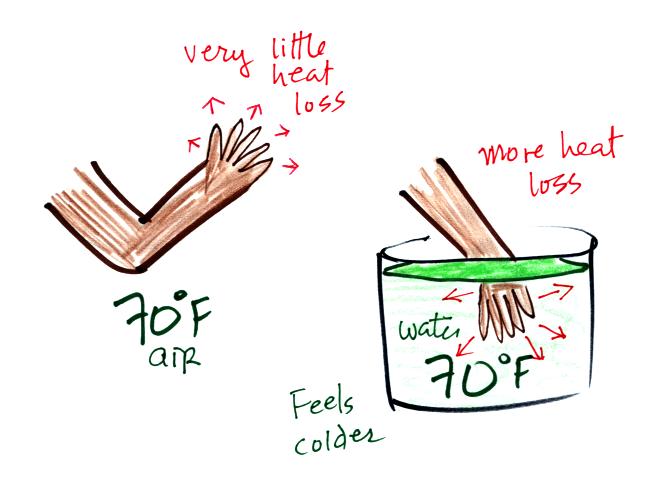
Here's a similar situation.
It's pleasant standing outside on a nice day in 70 F air. But if
you jump into 70 F pool water you
will
feel cold, at least until you "get used" to the water temperature (your
body might reduce blood flow to your extremeties and skin to try to
reduce energy loss).
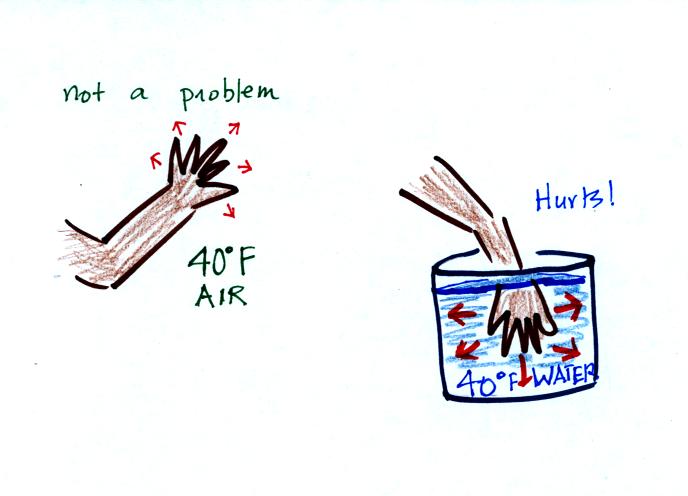
Air is a poor conductor. If you go out in
40 F
weather you will feel colder largely because there is a larger
temperature difference between you and your surroundings (and
temperature difference is one of the factors that affect rate of energy
transport by conduction).
If you stick your hand
into a bucket of 40 F water (I would suggest you give it a try
sometime), it will feel very cold (your hand will
actually soon begin to hurt). Water is a much better conductor
than air. Energy flows much more rapidly from your hand into the
cold water.
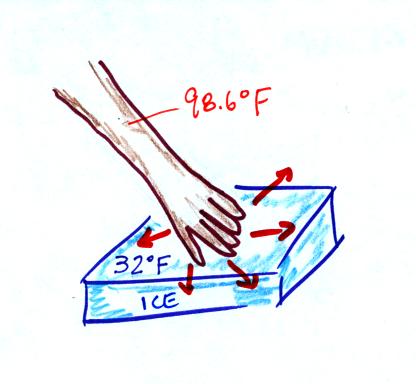
Ice
feels cold even though it is not a particularly good
conductor. This is because of the large temperature difference
between your hand and the water. The ice figure
wasn't shown in class.
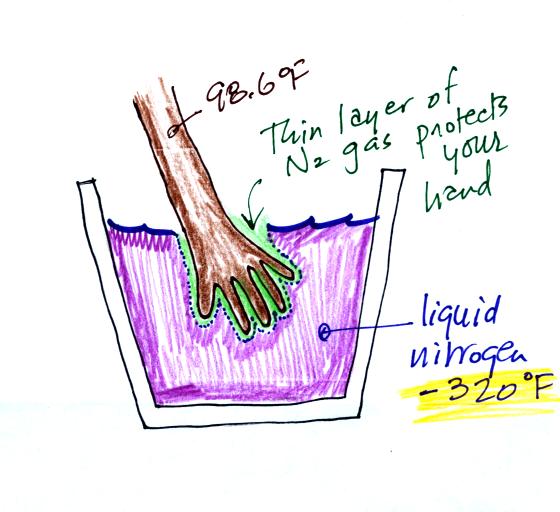
What about liquid nitrogen? It has a temperature
of
-320F! You can safely stick your
hand in liquid nitrogen for a fraction of a second. It doesn't
feel particularly cold and doesn't feel wet. Some of the liquid
nitrogen quickly evaporates and surrounds your hand with a layer of
nitrogen
gas. This gas is a poor conductor and insulates your
hand from the cold for a short time.
This basic knowledge puts us in a perfect position to understand
the
concept of wind
chill temperature.
Your body works hard to keep its core temperature around
98.6 F. If you go outside on a 40 F day (calm winds)
you will
feel
cool; your
body is losing energy to the colder surroundings (by conduction
mainly). Your body will be able to keep you warm for a little
while anyway (maybe indefinitely, I don't know). A thermometer
behaves differently, it is supposed to cool to the temperature of the
surroundings. Once it reaches 40 F it won't lose any additional
energy. If your body cools to 40 F you will probably die.
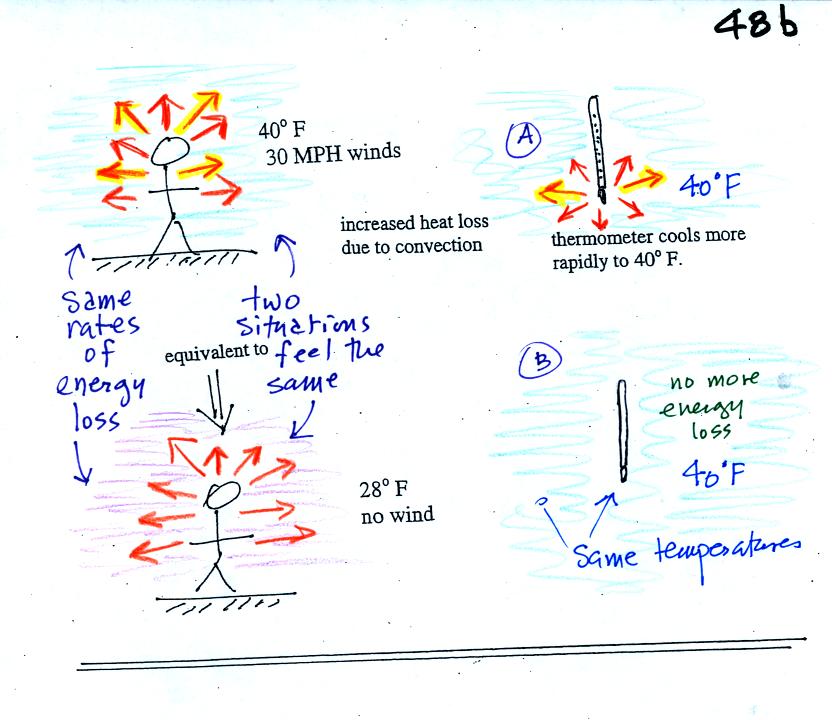
If you go outside on a 40 F day with 30 MPH winds your
body
will lose
energy at a more rapid rate (because convection together with
conduction are transporting energy away from your body). Note the
additional arrows drawn on the figures above indicating the greater
heat loss. This
higher rate of energy loss will make it feel colder
than a 40
F day
with calm winds.
Actually, in terms of the rate at which your
body loses energy, the windy 40 F day would feel the same as a 28
F day without any wind. Your body is losing energy at the same
rate in both
cases. The combination 40 F and 30 MPH winds results in a wind
chill temperature of 28 F.
The thermometer will again cool to the
temperature of its surroundings, it will just cool more quickly on a
windy day. Once the thermometer reaches 40 F there won't be any
additional energy flow. The
thermometer would measure 40 F on both the calm and the windy day.
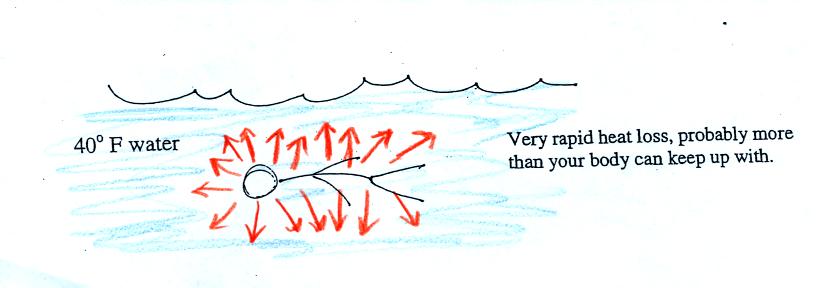
Standing outside on a 40 F day is not an immediate life
threatening
situation. Falling into 40 F water is.
Energy will be conducted away from your body more quickly
than
your
body can replace it. Your core body temperature will drop and
bring on hypothermia.
Be
sure
not
to
confuse
hypothermia
with hyperthermia
which
can bring on
heatstroke and which is also a serious outdoors risk in S.
Arizona.
At this
point I showed a
picture from the March 2005 issue of National Geographic. A Buddhist
monk was standing in a frigid waterfall. The caption for the
photograph read "To focus the mind and increase awareness of self,
Shingon Buddhists like Souei Sakamoto practice takigyo,chanting
for
hours
while
standing
in
frigid
waterfalls at the Oiwasan Nissekiji
Temple in Toyama, Japan." (I can't really scan the photograph and
include it in the classnotes because of copyright laws)
A second photograph from the December 2005 issue showed a monk hanging
from a tree by his feet. The caption there read "To
see
life
as
it
truly
is
- that's the goal of a student in China who
strengthens mind and body under the rigorous tutelage of a Shaolin kung
fu master."
Perhaps the most amazing example of a physical and mental task (not mentioned in
class) is the
1000-day challenge undertaken by the "marathon
monks" of Mount Hiei, Japan.
I hope you don't mind an
occasional digression like this. I spend a lot of time
riding my bicycle
uphills. It's not really painful but can definitely be
uncomfortable. I've
noticed that you can sometimes be distracted by a thought and ride a
mile or so and completely blank out the discomfort. With some
"Buddhist monk like" training I wonder if maybe I couldn't ride uphill
more or less indefinitely and not feel any discomfort at all.
This time of the year it is sometimes cold in the morning when I'm out
riding my bike. With some mental training
I hope be to be able to block out the feeling of
cold fingers and toes. I'm not there yet but will continue to
work on it.
Latent
heat energy transport was the next topic of the day.
Energy
transport in the form of latent heat is the second most important
energy transport process (second only to electromagnetic
radiation).
If you had an object that you wanted to cool off quickly you could blow
on it. Or you could stick it into some water, that would cool it
off pretty quickly because water will conduct energy more rapidly than
air. With a really hot object immersed in water,
you'd probably hear a brief sizzling sound, the sound
of boiling water. A lot of energy would be taken quickly from the
hot object and used to boil (evaporate) the water.
Latent heat energy transport is sometimes a little hard to visualize
or understand because the energy is "hidden" in water vapor or water.
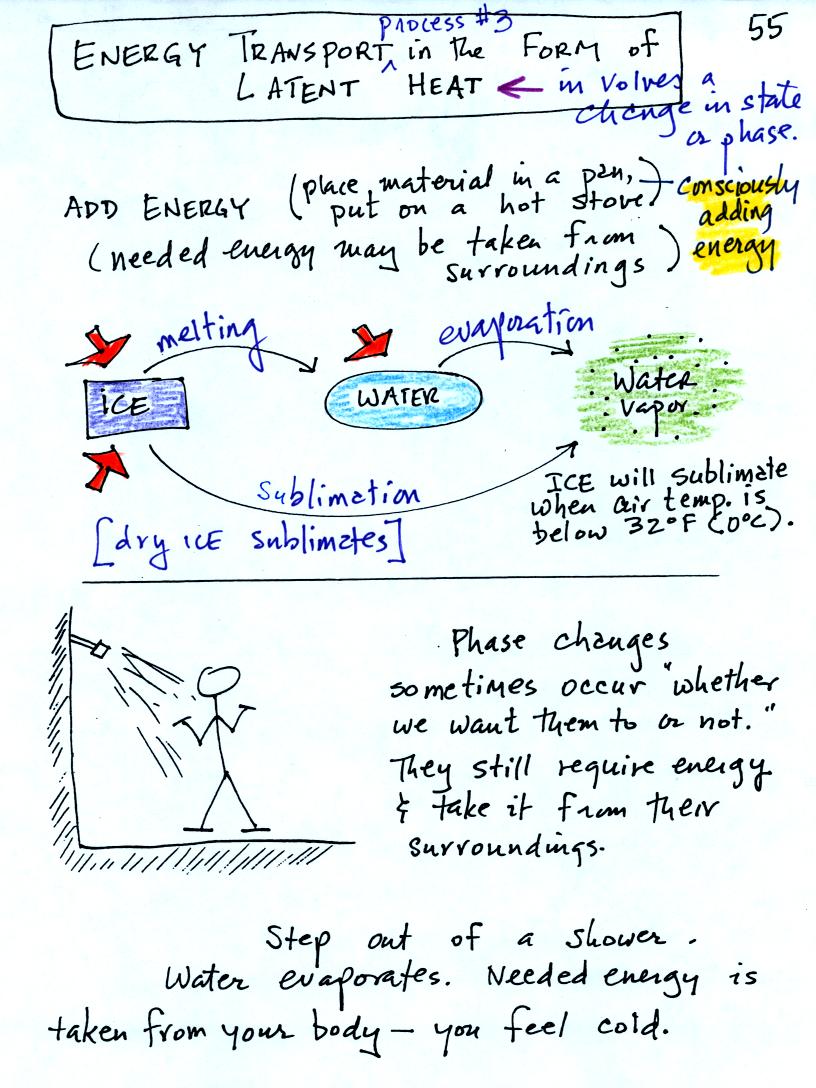
Latent heat energy transport is
associated with changes of
phase (solid to liquid, water to water vapor, that sort of thing) A
solid to liquid phase change is melting, liquid to gas is
evaporation, and sublimation is a solid to gas phase change (dry ice
sublimates when placed in a warm room, it turns directly from solid
carbon dioxide to gaseous carbon dioxide).
In
each case energy must be added to the material changing phase.
You can consciously add or supply the energy (such as when you put
water in a
pan and put the pan on a hot stove) or the phase change can occur
without you playing any role. In that case the needed energy will
be
taken from the surroundings. When you step out of the shower in
the morning, the water takes energy from your body and
evaporates. Because your body is losing energy your body feels
cold.
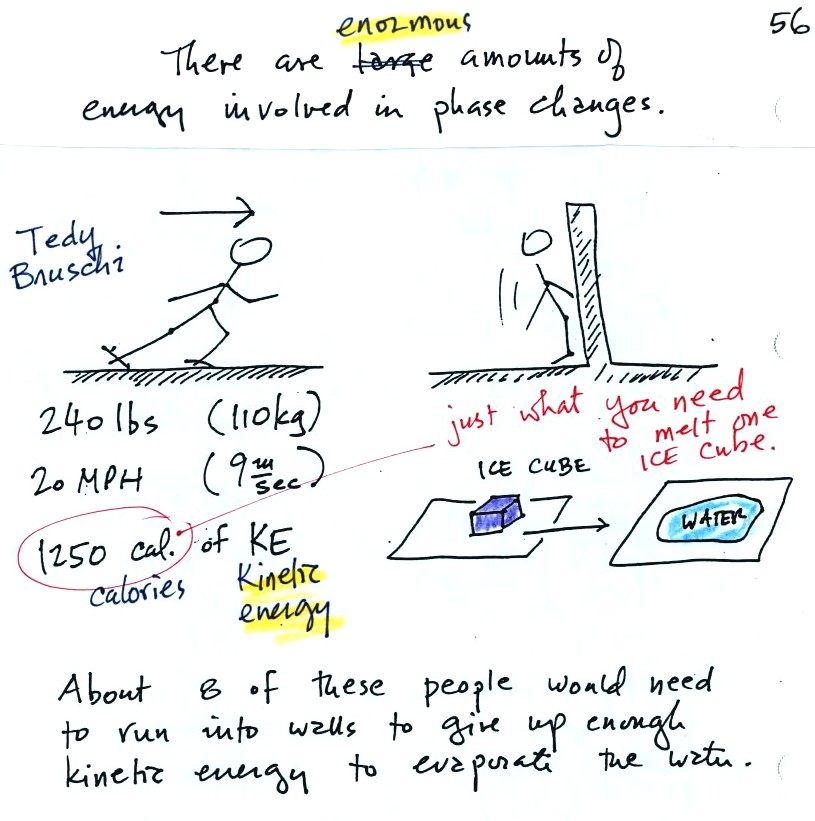
The object of this figure is to give you some appreciation for the
amount of energy involved in phase changes. A 240 pound man or
woman running at 20 MPH has just
enough
kinetic energy (if you could capture it) to
be able to melt an ordinary ice cube. It would take 8 people
running at 20 MPH to
evaporate the resulting water.
When you freeze water and make an ice cube energy is released into the
surroundings. You can picture the released energy as being a 240
lb person running at full speed.
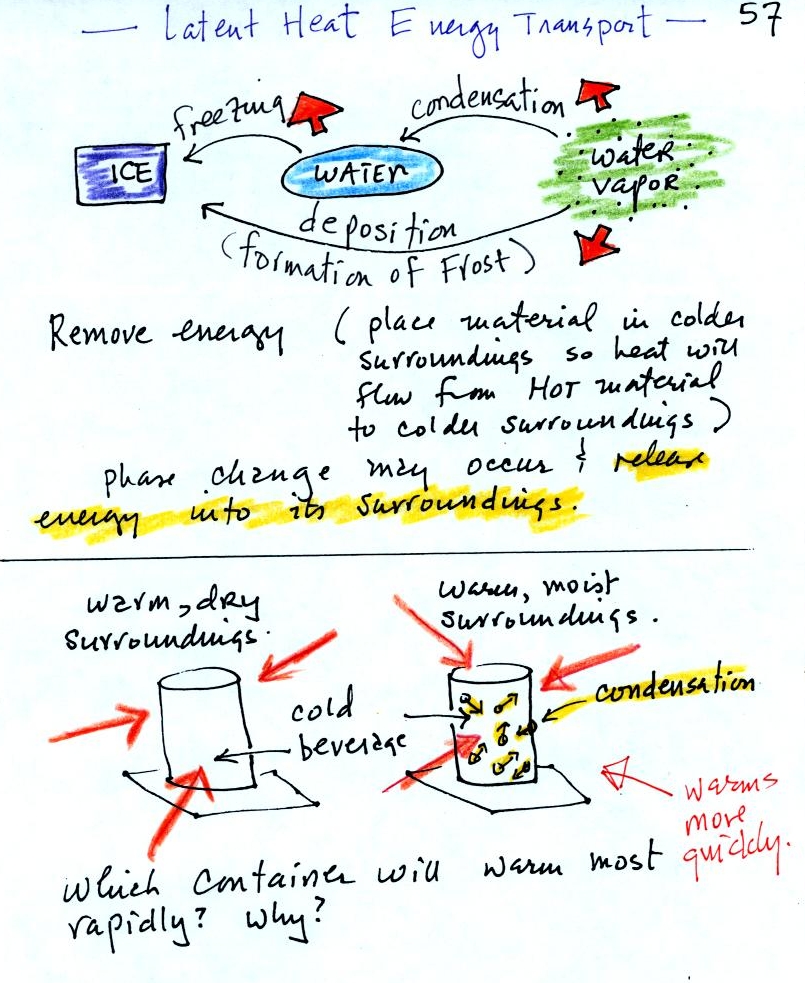
You can consciously remove energy from water vapor to make
it
condense
or from water to cause it to free (you could put water in a
freezer; energy would flow from the relatively warm water to the
colder surroundings). Or if one of these phase
changes occurs energy will be released into the surroundings (causing
the surroundings to warm). Note the orange energy arrows have
turned around and are pointing from the material toward the
surroundings.
A can of cold drink will warm more quickly in warm moist surroundings
than in warm dry surroundings. Heat will flow from the warm air
into the cold cans in both cases. Condensation of water vapor is
an additional source of energy and will warm that can more
rapidly. The condensation may actually be the dominant process.
You feel cold when you step out of a shower and water on
your body
evaporates. The opposite situation, stepping outdoors on a humid
day
and actually having water vapor condense onto your body (it can happen
to your sunglasses but not to you, your body is too warm). If it
did
happen it would warm you up.
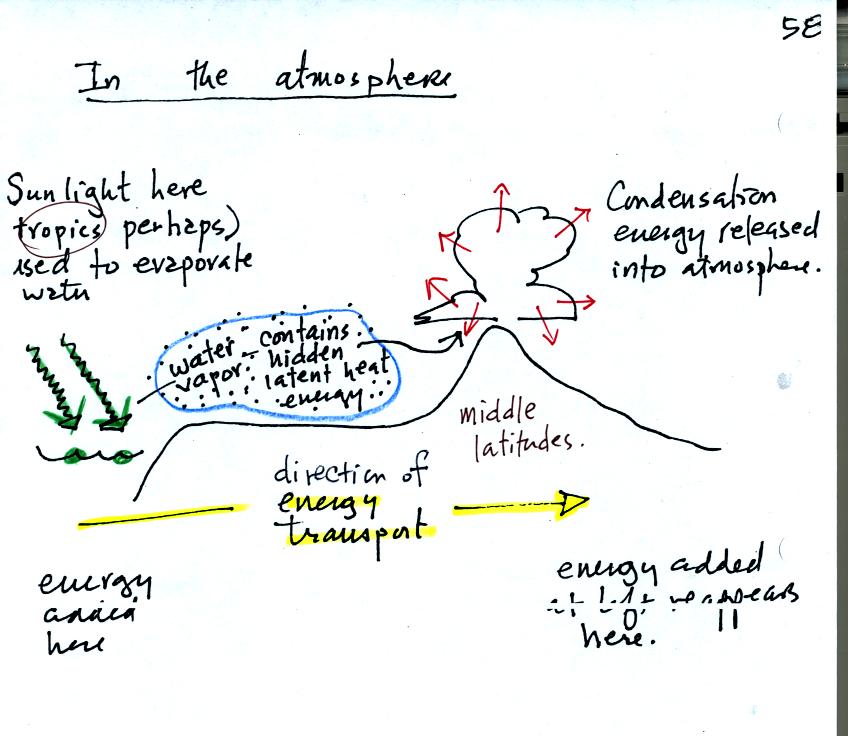
This figure shows how energy can be transported from one
location to another in the form of latent heat. The story starts
at left in the
tropics where there is often an abundance or surplus of sunlight
energy. Some of the incoming
sunlight
evaporates ocean water. The resulting water vapor moves somewhere
else and carries hidden latent heat energy with it. This hidden energy
reappears when something (air running into a mountain and rising,
expanding, and cooling) causes the water vapor to condense. The
condensation releases energy into the surrounding atmosphere.
Energy arriving in sunlight in the tropics has effectively been
transported to the atmosphere in Tucson.
We'll
spend probably the next couple of class periods on electromagnetic
(EM) radiation. It is the most important energy transport process
because it can travel through empty space. The notes that
follow are quite a bit more
detailed those written down in class.
To really understand EM radiation you need to know something
about electric
fields. To understand electric fields we need to quickly review a
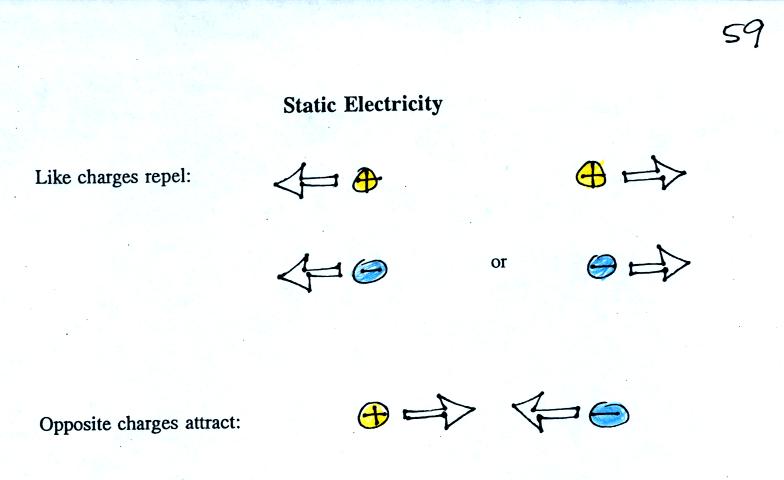
basic rule concerning static electricity.
These rules were
demonstrated using a wool sweater and a couple of balloons.
Each balloon was rubbed with the sweater. The
balloons (and the sweater) all became electrically charged (the
balloons
had one polarity of charge, the sweater had the other).
We
didn't
know
what
charge
the
balloons
carried just that they both had the same charge.
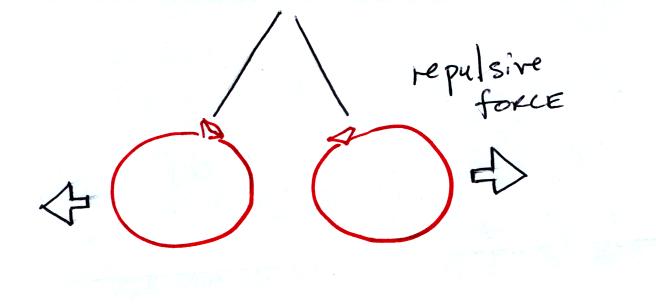
If you bring the balloons close to each other they are
pushed apart by
a repulsive electrical force.
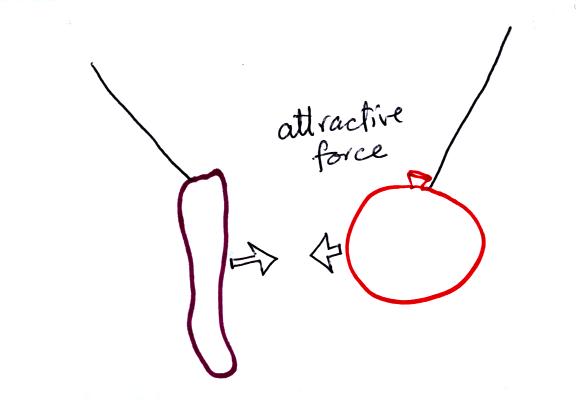
The sweater and the balloon carry opposite
charges. IF
they are
brought together they experience an attractive electrical force.
Our next step in understanding EM is to learn something
about electric
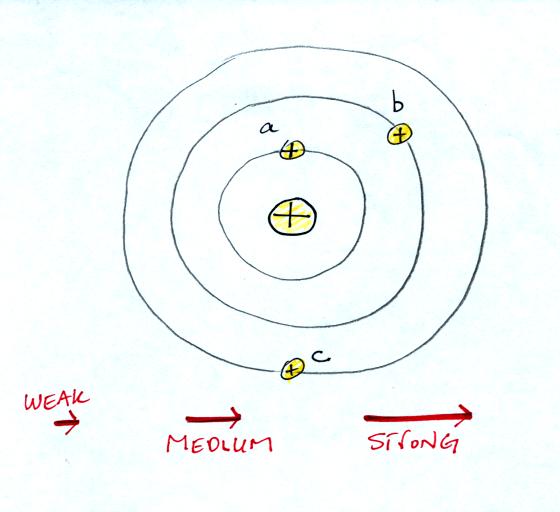
field arrows. Imagine placing a + charge at the three
positions shown in the figure
below.
Then choose one of the three arrows
at the bottom of the picture to show both the direction and the force
that would be exerted on each charge.
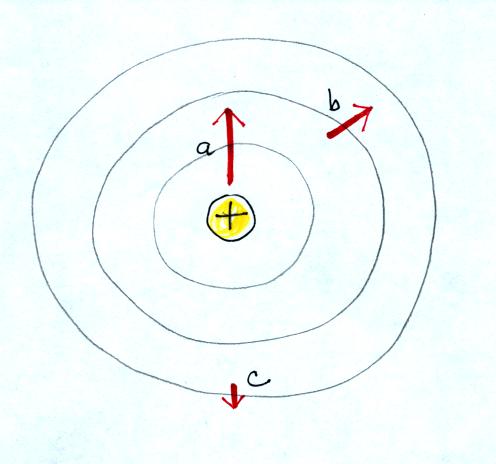
Here's the answer. The closer
the charge is to the center, the greater the strength of the outward
force. With just a little thought you can see that if you were to
place + charges at other
positions you would quickly end up with a
figure that looks like the pattern at the bottom of p. 59 in the
photocopied ClassNotes.
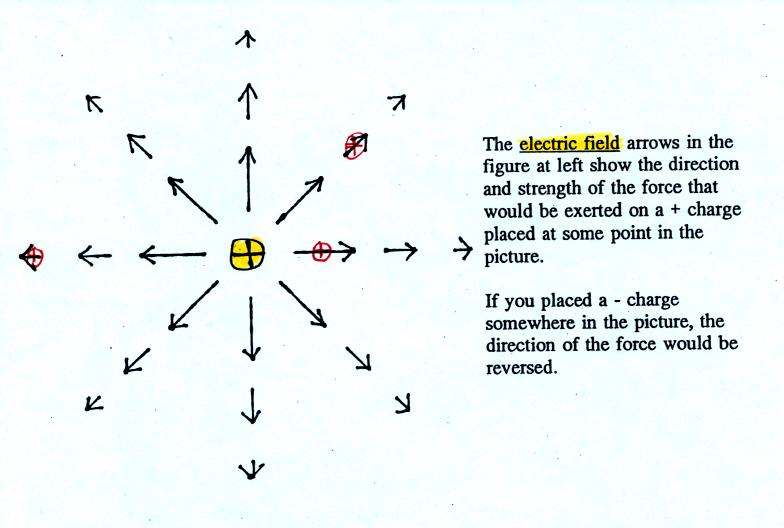
The electric field arrows in this
picture show the direction and give an idea of the strength that would
be exerted on a positive placed at any position in the figure.
You'll find the following on p. 60 in the photocopied ClassNotes.
I just hinted that this was the direction we would be going once we
come back to this topic next Tuesday. I'll put in some of the
details just in case you are reading ahead.
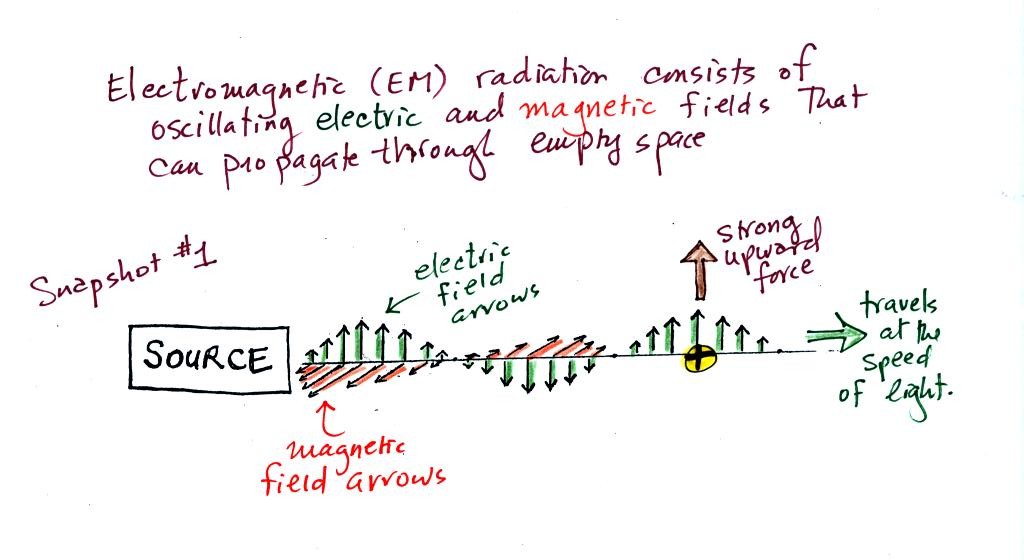
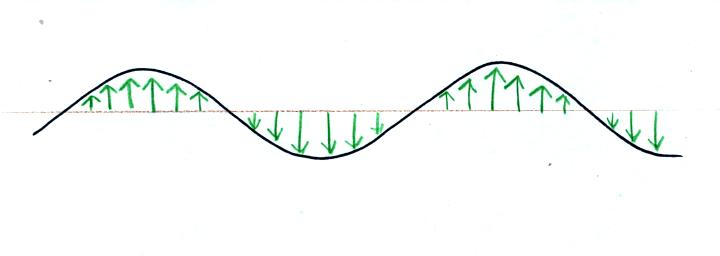
We imagine turning on a source of EM radiation and then
a
short time
later we take a snapshot. The EM radiation is a wavy pattern of
electric and magnetic field arrows. We'll ignore the magnetic
field lines. The E field lines sometimes point up, sometimes
down. The pattern of electric field arrows repeats itself.
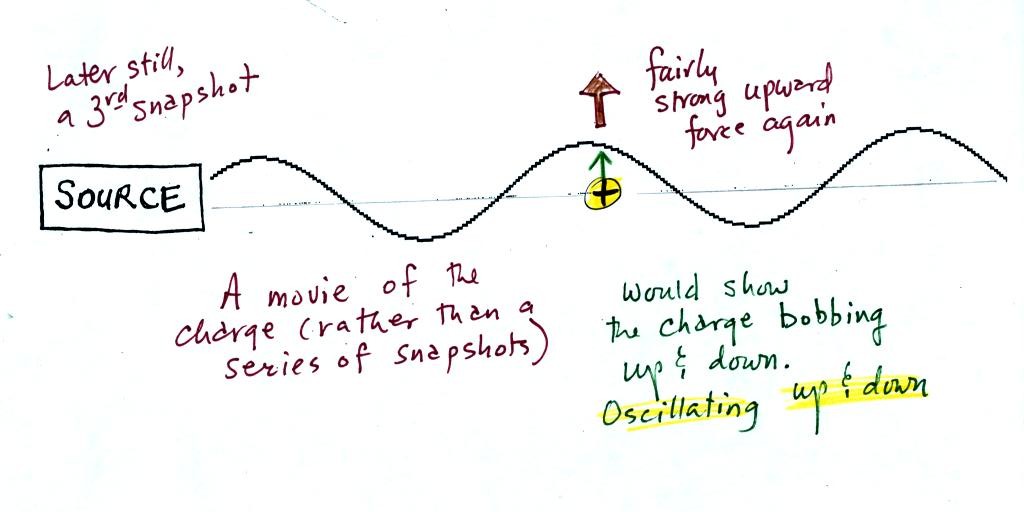
Note the + charge near the right
side of the picture. At the time
this
picture was taken the EM radiation exerts a fairly strong upward force
on
the
+
charge.
Textbooks often represent EM radiation with a wavy line like shown
above. But what does that represent?
The wavy line just connects the
tips of a bunch of electric
field
arrows.
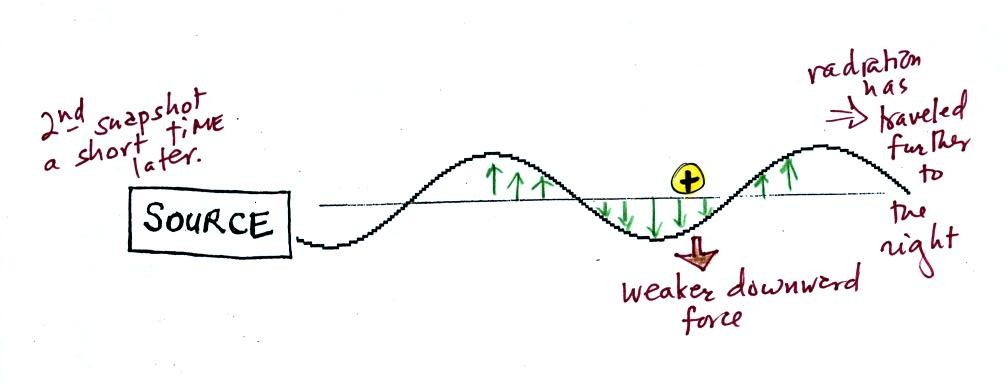
This picture was taken a short time after the first snapshot when
the radiation
had
traveled a little further to the right. The EM radiation now
exerts a somewhat weaker downward force on the + charge.
The + charge is now being
pushed upward again. A
movie
of
the +
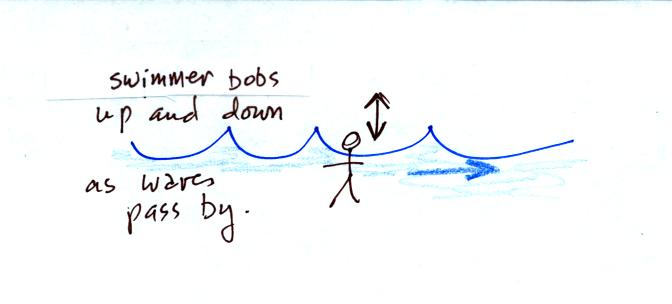
charge, rather than just a series of snapshots, would show the
charge
bobbing up and down much like a swimmer in the
ocean would do as waves passed by.
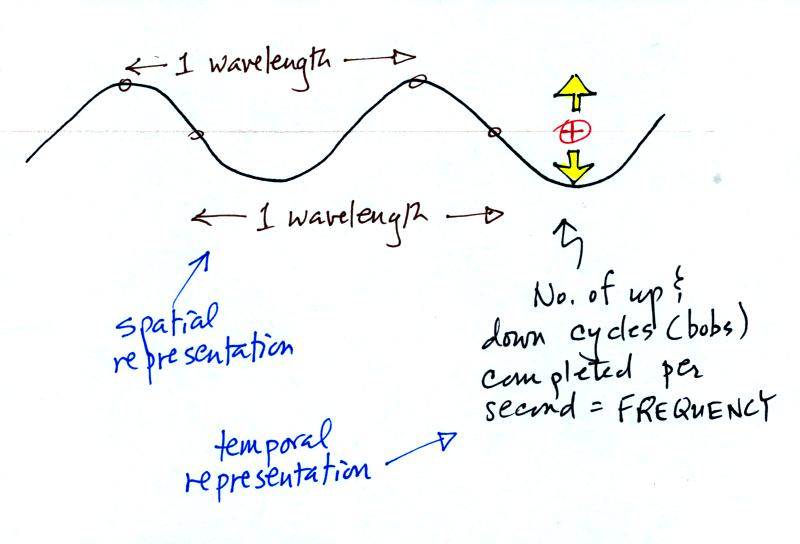
The wavy pattern used to
depict EM radiation can be described spatially in terms of its
wavelength,
the distance between identical points on the pattern. By
spatially we mean you look at different parts of the radiation at one
particular instant frozen in time.
Or you can
describe the radiation temporally
using the frequency of oscillation
(number of up and down cycles completed by an oscillating charge per
second). By temporally we mean you at one particular point for a
certain period of time.
In-class Optional Assignment
Question #1
The _______ is the amount of energy that you must add
to 1 gram of a material to raise its temperature 1 C.
a. density b. kinetic energy c.
latent heat d. specific heat e.
thermal conductivity
Question #2
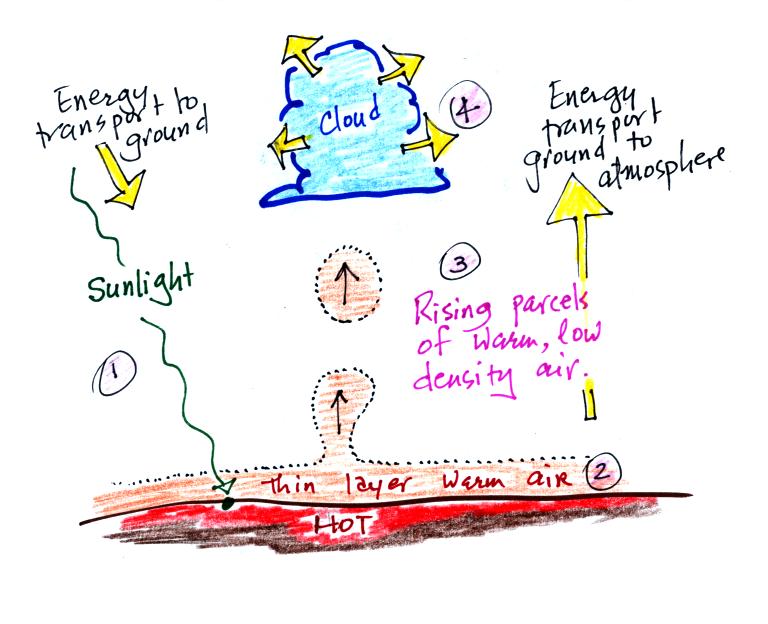
Try to identify the energy
transport
process shown at each of the numbered points in the picture above.