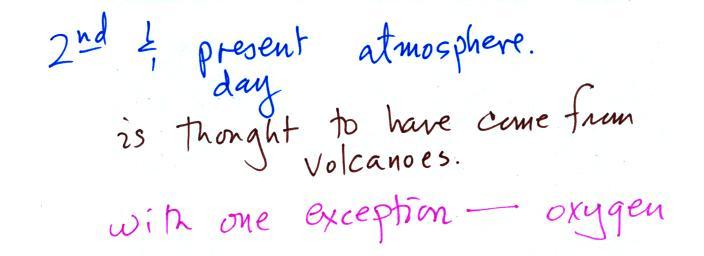This picture shows methane, aka
natural gas and a potent greenhouse gas, coming from melting
permafrost in Alaska (photo credit: Todd Paris/The
Associated Press. You'll find the photo in an
article that appeared in USA Today). You can
learn more about methane in a short video.
A little bit of additional material, not mentioned in class, was
stuck
onto the end of the online lecture notes from
last Wednesday. We
reviewed that quickly at the start of class today.
Then it was on to the main topic of the day. The origin and
evolution of our present day atmosphere.
The atmosphere we have today (mostly nitrogen,
oxygen, water
vapor, and argon) is very different from the earth's original
atmosphere which was mostly hydrogen and helium. This
original atmosphere either escaped (the earth was hot and the gases
were moving around with enough
speed that they could overcome the pull of the earth's gravity) or was
swept into space by the solar wind (click
on the link if you are interested in learning more about the solar
wind, otherwise don't worry about it).
With the important exception of oxygen, most of our present
atmosphere is though to have come from volcanic
eruptions.

Don't worry
about remembering all of the gases listed above. Volcanoes emit a
lot of
water vapor and carbon
dioxide. As
the earth began to cool the water vapor condensed and began to create
and fill oceans. Carbon dioxide dissolved in the oceans and was
slowly
turned into rock. Smaller amounts of nitrogen (N2) are also
emitted by
volcanoes. Because nitrogen is relatively unreactive it remained
in the
air and its concentration began to built up over time. There are
lots of poisonous gases such as sulfur dioxide emitted by
volcanoes. We'll learn
a little more about sulfur dioxide, in particular, next week when we
cover air
pollutants.
Two years ago, air travel to Europe
was being
severely disrupted by the eruption of the the Eyjafjallajökull volcano in
Iceland. Here are some really
amazing
pictures published in the Boston Globe.
Here's
another
set
of
photos also from the Boston Globe.
Volcanoes didn't add any of the oxygen that is the
atmosphere.
Where did that come from?
The oxygen is thought to have first
come from
photodissociation of
water vapor and carbon dioxide by ultraviolet (UV) light (the high
energy
UV light is able to split the H20
and CO2
molecules into
pieces). The O and OH then react
to form O2 and H.
By the way I don't expect you to remember
the chemical formulas in the example above. It's often easier and
clearer to show what is happening in a chemical formula than to write
it out in words. If I were to right the equations down, however,
you
should be able to interpret them. Ultraviolet is a high energy
form of light and it's probably also good to
remember that
ultraviolet light is capable of breaking molecules apart.
Once molecular oxygen (O2) begins to accumulate in the
air UV light can split it apart to make atomic oxygen (O). The
atoms of oxygen can react with molecular oxygen to form
ozone (O3).
Ozone in the atmosphere began to absorb ultraviolet
light and life forms could then begin to safely move from the oceans
onto land. Prior to the buildup of ozone, ocean water offered
protection from UV light.
Photosynthesis is the 2nd and
now
the
main
source of atmospheric oxygen.
Photosynthesis in its most basic form is shown in the chemical
equation above. Combustion is really just the opposite of
photosynthesis and is shown below.

We burn fossil fuels (dead,
undecayed plant material) to generate energy.
Water vapor and carbon dioxide are by products. Combustion is a
source of CO2.
We'll see these two equations again when we study the greenhouse
effect (CO2 is
a
greenhouse
gas
)
and
global warming.
The
following figure is the first page in the packet of photocopied
ClassNotes.
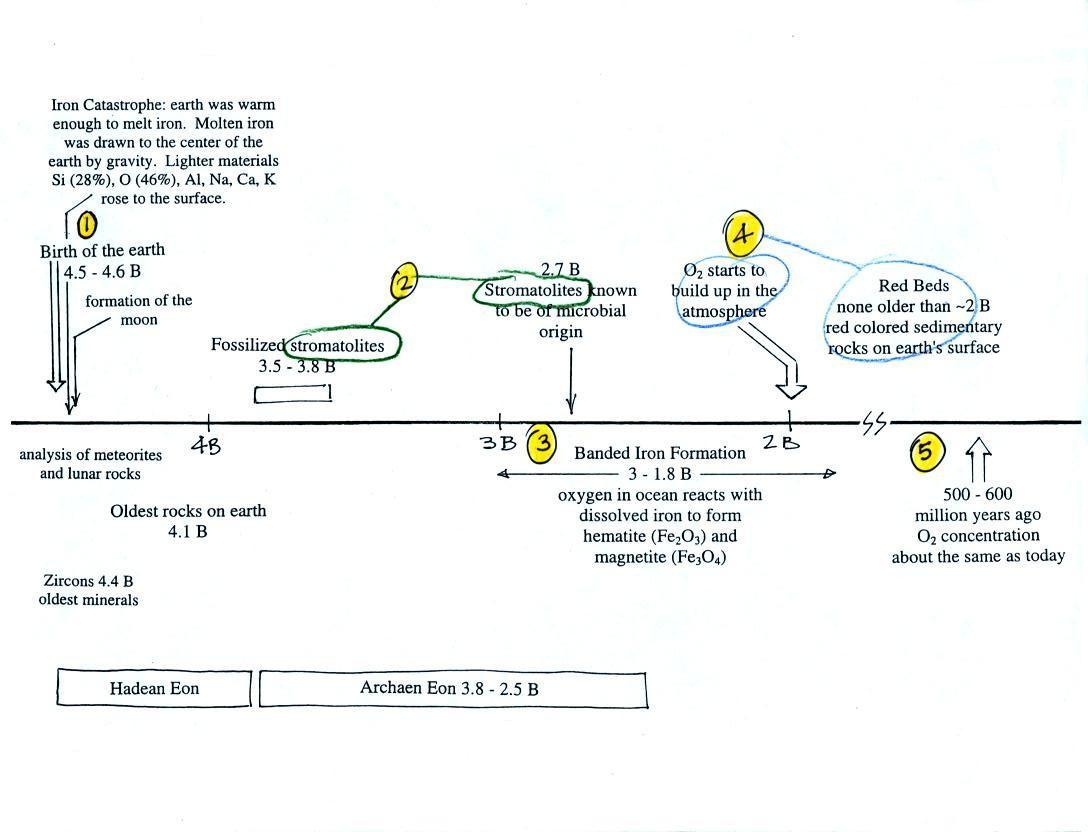
This somewhat confusing
figure shows some of the important events in the history of the earth
and evolution of the atmosphere. The numbered points were
emphasized.
First, Point 1: the earth
is thought to be between 4.5
and 4.6 billion years old. If you want to remember the earth is a
few
billion years old that is probably close enough. Something I didn't
mention in class, it's in small type above. The formation
of a molten iron core was important because it gave the earth a
magnetic field. The magnetic field deflects the solar wind and
keeps it from blowing away our present atmosphere.
Stromatolites
(Point
2)
are
column-shaped
structures
made
up of layers of sedimentary rock, that are created by
microorganisms
living at the top of the stromatolite (I've never actually seen a
stromatolite, so this is all based on photographs and written
descriptions). Fossils of the very small microbes (cyanobacteria
= blue green algae)
have been found in stromatolites as old as 2.7 B years and are some of
the earliest records of life on earth. Much older (3.5 to 3.8
B years old) stromatolites presumably also produced by microbes, but
without
microbe fossils, have also been found.

Blue green algae grows at the top
of the column, under water but near the ocean surface where it can
absorb
sunlight. Sediments begin to accumulate on top of the algae and
start to block the sunlight. The cyanobacteria would then move to
the top of this sediment layer and the process would repeat
itself. In this way the stromatolite
column would grow a layer at a time. We're learning about
stromatolites
because the cyanobacteria on them were one of the earliest forms of
life able to produce oxygen using
photosynthesis.
Living stromatolites are found in a few
locations today. The two pictures above are from Coral Bay (left)
and Shark's Bay (right) in Western Australia. The picture was
probably taken at
low tide, the stromatolites would normally be covered with ocean
water. It doesn't look like a good place to go swimming, I would
expect the top surfaces of these stromatolites to be slimy.
Point
3 refers to banded iron rock. These rocks are 3 billion
years old (maybe older) and are evidence of oxygen being produced in
the earth's oceans. Here are a couple of pictures of samples of
banded iron formation rock that was passed around in
class.
The main thing to notice are the alternating
bands of red and black
rock. The next paragraph and figure explain how these formed.
Rain would first of all wash iron ions from the earth's land surface
into the ocean (at a time before there was any oxygen in the
atmosphere). Oxygen from the cyanobacteria living in the ocean
water reacted with the dissolved iron (the iron
ions) to form hematite or magnetite. These
two minerals precipitated out of the water to form a layer on the sea
bed. This produced the black layers.

We finished with a question from last semester's final exam:
 Equation (c) is the first step in the natural production of
ozone. This is followed by the reaction in Eqn. (a).
Together Eqns. (c) and (a) produce ozone. Equation (b) shows the
natural destruction of ozone.
Equation (c) is the first step in the natural production of
ozone. This is followed by the reaction in Eqn. (a).
Together Eqns. (c) and (a) produce ozone. Equation (b) shows the
natural destruction of ozone.