Quiz #2 Study Guide
Energy,
temperature and heat (20-25 pts). Kinetic energy -
energy of motion. Temperature (which scale?) provides a
measure of the average kinetic energy of the atoms or molecules in
a substance. Energy units: calories. What is the
relationship between energy added to (or removed from) an object,
ΔE, and the temperature
change, ΔT, that
results? Specific heat (aka thermal mass or thermal
capacity). Water has a relatively high specific heat (4 or 5 times
higher than soil). A city on a coastline will have a more
moderate climate (what does that mean?) than a city located
further inland. Other than a change in temperature what else
can happen when energy is added to or removed from a material?
Temperature scales (15 pts).
Fahrenheit, Celsius, and Kelvin (absolute) scales.
You should know the temperatures of the boiling point of water at
sea level and the melting point of ice (same as the freezing point
of water) on the F and C scales. The global average surface
temperature of the earth is about what temperature on the Kelvin
scale?
Energy transport (15-20
pts).
(1) Conduction. Energy is
transported from hot to cold by random atomic or
molecular motions at a rate that depends on the material
(thermal conductivity) and the temperature gradient.
Examples of good and poor conductors. An object with
high thermal conductivity will often feel cold to the
touch because it rapidly conducts energy away from your
body (our perception of temperature is an indication of
how quickly our body is losing energy, not a good
measurement of temperature).
(2) Convection. Energy transport by organized motion of
atoms or molecules (works in gases and liquids but not
solids). Free (rising and sinking air) and forced
convection. Free convection is a third way of causing
rising air motions in the atmosphere (what are the other
two?). Wind chill temperature.
Energy
transport (25 pts).
(3) Latent heat energy
transport. 2nd most important energy transport
process. Six phase change names. For each phase change
you should know whether energy is added to a material
(absorbed from or taken from the surroundings) or taken from
the material (released into the surroundings).
Sample
Questions
Quiz #1: 5, 12, EC3 Final Exam: 12,
25, 43, 53 See also this extra set of Sample
Questions
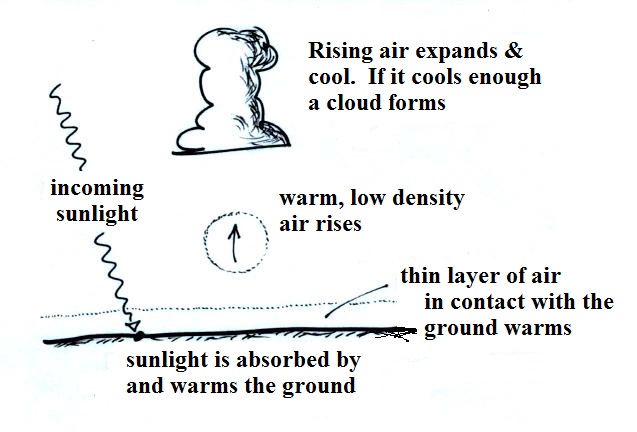
All 4 energy transport processes are at work
in this figure.
Static electricity and electric
fields (5 pts).
Like charges repel, opposite charges attract. An electric
field arrow shows the direction and strength of the force that
would be exerted on a positive charge placed at that location.
Electromagnetic radiation (15
pts). The most important of the
4 energy transport processes (why?). Oscillating electric and
magnetic fields that can propagate (at the speed of light) through
empty space (and also transparent materials like glass & air).
Radiation can be produced by moving charges. You add energy to
cause the charges to oscillate and produce the radiation. Energy
reappears when the resulting radiation causes electrical charges
somewhere else to move. Wavelength is one way of distinguishing
between different types of radiation (frequency is another). Would
a slowly-oscillating charge produce long- or short-wavelength
radiation? Would this be a relatively high- or low-energy form of
radiation? Electromagnetic spectrum. We will mostly be concerned
with ultraviolet (UV), visible (VIS), infrared (IR) light. What is
the wavelength interval for visible light? What is white light?
Does red light have longer, shorter, or the same wavelength as
blue light? Wavelength units.
Rules governing
the emission of radiation (15 pts). What determines how
much and what type of radiation an object will emit (the same
variable is found in both the Stefan-Boltzmann law and Wien's
law)? A light bulb connected to a dimmer switch was used to
demonstrate. Radiant energy emitted by the earth (300 K) and sun
(6000 K).
Radiative
equilibrium
(10 pts). Energy balance.
Incoming radiant energy (sunlight) is balanced by an equal amount
of (but not necessarily the same kind of) outgoing radiant energy,
temperature remains constant.
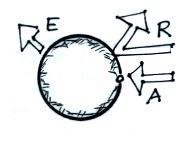 What would the relationship be between A(absorption),
E(emission), and R(reflection) when this planet
is in radiative equilibrium
What would the relationship be between A(absorption),
E(emission), and R(reflection) when this planet
is in radiative equilibrium
Filtering effect of the
atmosphere (15 pts). Does the atmosphere mostly absorb,
selectively absorb, or mostly transmit UV, VIS, and IR radiation?
What gases are important in each case? What does the term window
mean? What property makes water vapor, carbon dioxide, methane,
etc. greenhouse gases?
Greenhouse
effect (simplified view) (10 pts). With
an atmosphere (containing greenhouse gases), the temperature of
the earth's surface is warmer than it would be without an
atmosphere. H2O, CO2, and other greenhouse
gases selectively absorb IR radiation. The atmosphere in turn
radiates IR radiation into space and back toward the ground. How
is it possible for the earth's surface to radiate away more energy
than it receives from the sun and still be in energy
balance?
Misc. energy balance questions
(20 pts). What effects do
clouds have on nighttime and daytime temperatures? Why?
What percentage of the sunlight arriving at the
top of the atmosphere reaches the ground and is absorbed?
What happens to the rest of the incoming sunlight energy?
Sample Questions
Quiz
#2: 2, 7, 8, 11, 12, 13, 14, 15, EC3
Final Exam: 15,
36 and see also this 2nd set of
Sample Questions
Reviews
Mon., Mar. 7
|
4:00 - 5:00 pm
|
Modern Languages 311
|
Tue., Mar. 8
|
4:00 - 5:00 pm
|
Haury(Anthropology) 129
|


