![[Home]](../../icons/home.jpg)
![[Lectures]](../../icons/lectures.jpg)
![[Previous]](../../icons/previous.jpg)
![[Next]](../../icons/next.jpg)
Ozone in the Atmosphere ; Chemical Destruction of Ozone in the Statosphere
Ozone is very rare in our atmosphere, averaging about three
molecules of ozone for every 10 million air molecules. In spite of
this small amount, ozone plays a vital role in the atmosphere.
Ozone is a form of oxygen
that comprises three atoms (O3) rather than
the two atoms (O2) found in ordinary
molecular oxygen.
 |
Ozone is mainly found in two regions of
the Earth's atmosphere. Most ozone (about 90%) resides in a layer
between 20 and 30 kilometers (12 and 18 miles) above
the Earth's surface.
This region of the atmosphere is part of the
stratosphere. Within the stratosphere, the
level of maximum concentration is at about 25 km (15
miles) where the average ozone concentration is 10 ppm (parts per million); that is, for every one
million molecules, 10 are ozone molecules.
The ozone in this region is commonly known as the
ozone layer.
It is important to realize that although
we refer to this region of higher ozone concentrations as the ozone layer,
ozone makes up only a small percentage the gas
molecules in ozone layer (10 out of 1 million). The fact that there is so
little ozone naturally in the stratosphere is one reason we are concerned
about human activities that deplete stratospheric ozone, as this ozone is
beneficial for life.
The other region of elevated ozone concentration happens just above
the ground surface and is mainly the result of human activity. This ozone
can be harmful and is considered pollution.
The figure above shows
how ozone is typically distributed in the atmosphere.
The ozone molecules in the upper atmosphere (stratosphere) and
the lower atmosphere (troposphere) are chemically identical
because they all consist of three oxygen atoms and have the
chemical formula O3. However, they have very
different roles in the atmosphere and very different effects on
humans and other living beings. Stratospheric ozone (sometimes
referred to as "good ozone") plays a beneficial role by absorbing
most of the biologically damaging ultraviolet sunlight (called
UV-B), allowing only a small amount to reach the Earth's surface.
The absorption of ultraviolet radiation by ozone creates a source
of heat, which actually forms the stratosphere itself (a region in
which the temperature rises as one goes to higher altitudes).
Ozone thus plays a key role in the temperature structure of the
Earth's atmosphere. Without the filtering action of the ozone
layer, more of the Sun's UV-B radiation would penetrate the
atmosphere and would reach the Earth's surface. Many experimental
studies of plants and animals and clinical studies of humans have
shown the harmful effects of excessive exposure to UV-B
radiation.
Because ozone is toxic to humans and many other animals and plants,
ozone near the ground surface is sometimes referred to as "bad ozone".
Naturally, very little ozone is present near the Earth's surface.
But, due to human activities, harmful concentrations can develop
under certain conditions.
The process that creates ozone near the ground is known as photochemical smog.
It occurs when vehicle exhaust, other industrial chemicals, and abundant
sunlight mix together in a complicated series of chemical reactions.
This can be a very big problem in large cities in the summertime.
Los Angeles, Phoenix, and Washington D.C. are examples of cities
where ozone concentrations sometimes reach dangerously high levels.
The national weather service will issue ozone warnings when ozone
levels become dangerously high near the ground. The rest of this
page concerns only stratospheric ozone and the issue of possible depletion
of stratospheric ozone due to human activity.
Summary for Stratospheric Ozone Depletion
- We know that certain manmade chemicals, CFCs several other molecules, such
as carbon tetrachloride, methyl bromide, methly chloroform, and halons,
chemically deplete the amount of ozone
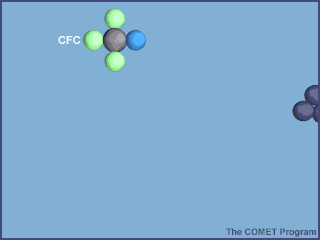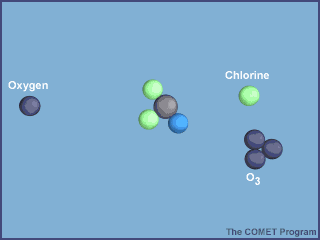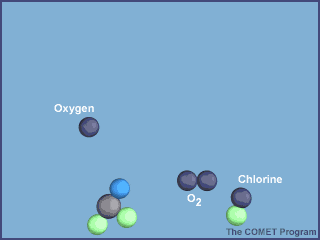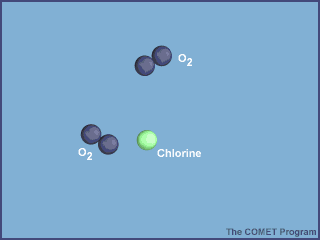
in the stratosphere. CFC stands for chlorofluorocarbon. A CFC is a methane molecule
(CH4, which is a central carbon atom with 4 hydrogen atoms attached) that has
one or more chlorine and or fluorine atoms substituted for the hydrogen atoms.
More generally, ozone-depleting chemicals contain chlorine
and/or bromine. When
chlorine and bromine atoms break free from these molecules in the stratosphere, they can destroy ozone.
Because these manmade molecules are chemically unreactive, once released, they remain in
the atmosphere for an average of 100 years. Eventually, they are lifted
from the lower troposphere into the stratosphere. Once in the stratosphere, they are
exposed to high levels of ultraviolet radiation. This breaks up the molecule
and releases chlorine and bromine atoms, which then destroy ozone in a subsequent
chemical reaction. Although the amount of chlorine and bromine in the stratophere is
quite low, alarmingly each free chlorine and bromine atom may be capable of destroying up
to 100,000 ozone molecules. Here is a link to a picture showing
A simplified chemical reaction pathway
describing how a CFC molecule
relaeses chlorine leading to ozone destruction.
The animation below shows the destruction of an ozone molecule by a chlorine atom, which was
liberated from a CFC. Note that the chlorine atom is freed in a subsequent reaction, allowing
it to chemically destroy more ozone molecules.
 |
- Summary of the observed loss of stratospheric ozone for different latitude zones:
- Tropical Latitudes (30°North to 30°South): Little, if any, changes observed
- Middle Latitudes (30° to 60° in both Northern and Southern Hemisphere): 6% decrease from 1980 to 1996, with slight increase after 1996
- Arctic Latitudes (60°North to North Pole): Reduction of ozone highly variable from year to year. Can spike to 30% reduction during cold periods in winter and spring
- Antarctic Latitudes (60°South to South Pole): Region where seasonal ozone "hole" occurs. The ozone hole is described in the next section
- Except for the ozone hole described below (and during extremely cold periods in the Arctic), ozone is destoyed in
homogeneous chemical reactions (gas phase chemical reactions) between
chlorine (or bromine) radicals and ozone. CFCs and other manmade chemicals provide the chlorine
or bromine radicals.
Depletion of ozone via homogeneous chemistry is relatively slow.
- Ozone "hole"
- Large depletion of stratospheric ozone (up to 70%) that occurs ONLY over
Antarctica from late September through early December (springtime in the
Southern Hemisphere). The meteorological conditions and other factors required for this
rapid destruction of ozone only happen in the Antarctic stratosphere in Southern
Hemisphere spring.
- Although often referred to as the
ozone 'hole', it is really not a hole but rather a
thinning or a depletion of a large amount of the ozone in the stratosphere. The term 'hole' in used in reference to
the large seasonal loss of stratospheric ozone over the southern pole, not complete removal of all ozone.
- The Antarctic ozone hole has been observed every
year since 1985. The geographical size and amount of the ozone depletion within
the ozone hole vary from year and depends somewhat on specific atmospheric conditions
in addition to the amount of free chlorine and bromine present.
- It has been proven that CFCs (and similar molecules) are responsible.
- Result of complex chemical reactions that can only happen under the extremely cold
conditions that develop in the Antarctic stratosphere. It is a heterogeneous
chemical reaction because some of the chemical reactions take place on the
surfaces of ice crystals. These ice crystals make up
polar stratospheric clouds, which only form when air temperatures drop below
about -90°F. This chemical pathway leads to very rapid destruction of ozone.
- Once the Antarctic stratosphere warms by December, ozone levels return to near
normal levels.
- Discovery of the ozone hole is what scared the developed world to take action
on the statospheric ozone depletion problem. While it was previously known that certain
manmade chemicals were capable of destroying stratospheric ozone, it was not realized
that destruction of stratopheric ozone could take place so quickly under the conditions
of the Antarctic stratosphere.
- A much smaller and weaker ozone "hole" has been observed over the Arctic region over short periods of time in years when it is cold enough
for the formation of extensive polar stratospheric clouds, but the
conditions necessary for rapid, sustained ozone depletion do not happen in the arctic stratosphere as they
do in the antarctic stratosphere.
- Montreal Protocol (1987) -- Developled countries agreed to phase out use of CFCs
and other ozone-depleting chemicals in World treaty.
Developed countries have almost completely substituted "ozone-friendly" chemicals
for CFCs since mid 1990s; there are still a few ozone-depleting chemicals that are
in the process of being phased out over the next decade or so in accordance with
international agreements. The figure below shows the sharp decline in the consumption
of known ozone-depleting substances from 1989 to 2009.
![[o3comp]](ozconsump.jpg) |
| The worldwide consumption (use) of ozone depleting products (ODP) in millions of tonnes from
1989 to 2009. There has been a sharp decrease after the Montreal Protocol. |
- It is expected that the ozone layer will fully recover, but it will take decades due to CFCs already
in the atmosphere and leaking CFCs from old style refrigeration systems. (Keep in mind that the
average lifetime of a CFC molecule in the atmosphere is about 100 years). In fact we are beginning to
see evidence that chlorine levels in the stratosphere are decreasing (see blue line in figure below on the left side, which corresponds
with the axis on the right side of the graph).
The figure on the left also indicates that the total loss of ozone each year (during the ozone hole period) has peaked and is
trending down. The figure on the right shows that the size of the ozone hole each year has stabalized since
the late 1990s and is showing signs of getting smaller. The size is defined as the area of the region that has ozone levels
below a minimum threshold.
![[Cl and O3]](ozone_recovery_700.jpg)
| ![[ozone]](ozone_hole_size_2012.jpg)
|
| Red line is observed ozone mass deficit ("missing ozone") and green line is
the projected ozone mass deficit over south polar region at time of minimum ozone.
The blue line (axis on right) shows the measured and projected changes in stratospheric chlorine content. |
Maximum size of ozone hole over south pole each year from 1980 to 2012. |
- We should expect to continue to observe an Antarctic ozone hole each year for at least decades. The
size of the ozone hole and the amount of ozone destoyed varies quite a bit from year to year
because the atmospheric conditions in the Antarctic winter and early spring are different
from year to year. However, it is expected that the long-term trend will be toward
a smaller ozone hole and less ozone depletion in the Antarctic. The figure above indicates that the
size of the Antarctic ozone hole has stabalized recently. World-wide measurements are showing that
the ozone layer is recovering, although full recovery to pre-industial levles of stratospheric ozone
is not expected until around the middle of this century as indicated by the green line in the
figure on the left side above.
Recent measurements indicate that
the size of the ozone hole continues to show signs that it is slowly getting smaller, although there
is quite a bit of variability from year to year as the size and duration of the ozone hole depends
on the specific atmospheric conditions in the Antarctic stratosphere each year. Here is a link to a
NASA video that claims large ozone holes over Antarctica will stop forming by 2040,
Big Ozone Holes Headed For Extinction By 2040.
- Despite all the doom and gloom articles that have been written about ozone depletion, most likely
none of us has been exposed to dangerously high levels of ultraviolet radiation due to ozone depletion.
Your risk of developing uv-related problems (such as skin cancer) depends more on your lifestyle, i.e., the
amount of sun exposure and use of sunscreens, than on the
rather small ozone depletion that has occurred up until now. In fact a 2010 report by the science
advisors to the Montreal Protocal, report that in middle latitudes, surface ultraviolet radiation has been
about constant over the previous decade from 2001 to 2010.
- In spite of all that is reported on this page, the incidence of both melanoma and melanoma skin cancers
has been increasing over the past decades in the United States and throughout much of the world.
Occasionally you will see someone trying to link that increase with human caused stratospheric ozone depletion.
However, this has little if anything to
do with stratospheric ozone depletion. Again, the main factors that predispose to the development of melanoma seem
to be connected with excessive exposure to the sun and a history of sunburn. These factors lie within each
individual's own responsibility. There are other contributing reasons for the increase in reported cases of
skin cancers. One is simply due to the fact that people live longer today and this increases the chance
that they will develop skin cancer. Another reason is that we have better detection and screening for skin cancer
as well as public education campaigns about the importance of early detection, which increases the number
or reported cases.
- The international agreements do show that the world is capable of dealing
with a global scale environmental threat, resulting from advanced technology. In this
case, the cause of ozone destruction was easily proven and substitutes for CFCs were
easily found without much additional cost. However, it remains to be seen if something
similar will occur with greenhouse gas emissions and global warming, where we do not have
a clear cause and effect mechanism nor a cheap alternative (to burning fossil fuel).
If you are interested, more detailed information about ozone depletion and the Antarctic ozone hole is
provided by
US Environmental Protection Agency Ozone Home Page.
![[Home]](../../icons/home.jpg)
![[Lectures]](../../icons/lectures.jpg)
![[Previous]](../../icons/previous.jpg)
![[Next]](../../icons/next.jpg)


![[o3comp]](ozconsump.jpg)
![[Cl and O3]](ozone_recovery_700.jpg)
![[ozone]](ozone_hole_size_2012.jpg)