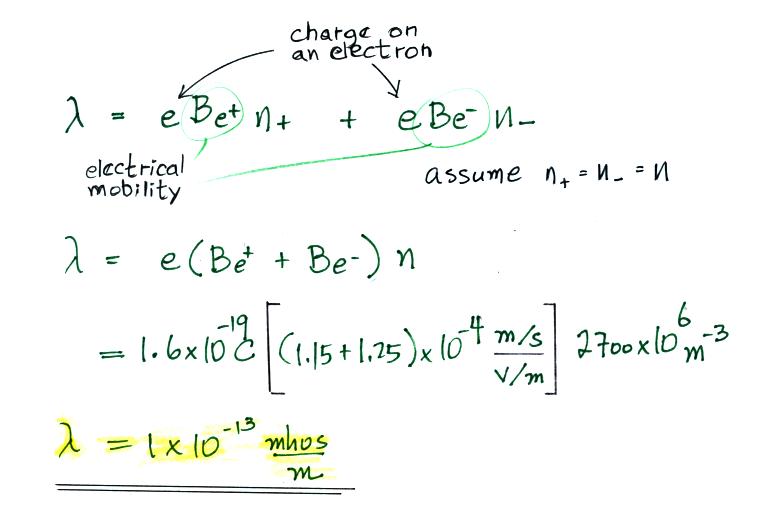
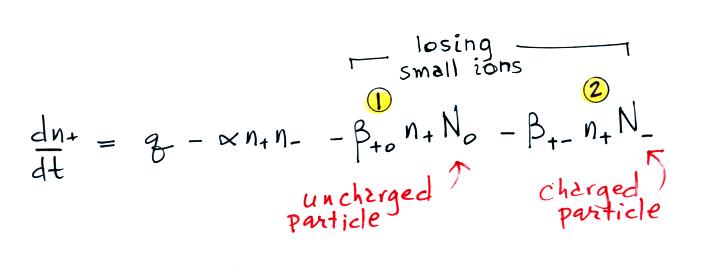
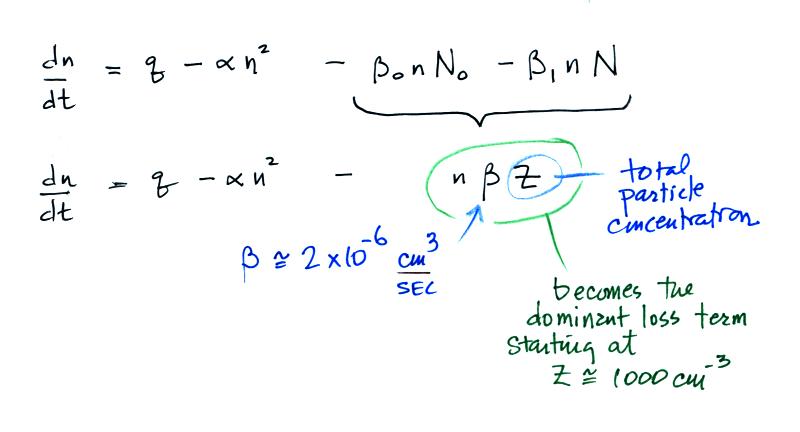
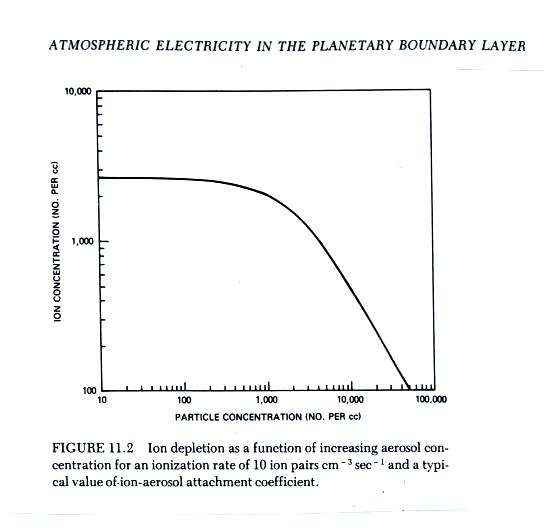
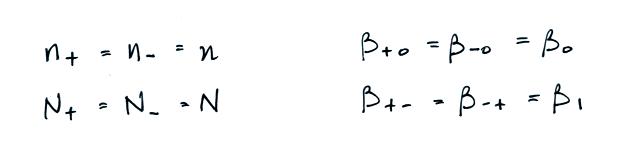Most smaller particles are uncharged.
The chart shows that roughly 90% of particles with a diameter
of 10 cm are uncharged. Small particles that
are charged generally only carry one electron charge.
For larger particles the numbers of particles that are
uncharged, positively charged, and negatively charged are
roughly equal. I.e. 1/3 of large particles are
positively charged, 1/3 are negatively charged, and 1/3 are
uncharged.

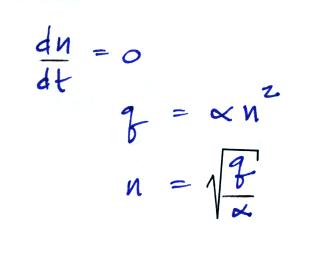
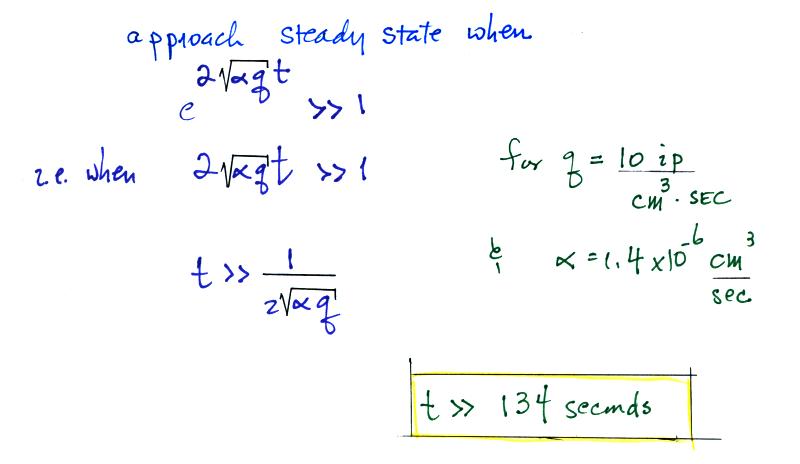
 .
.