Friday Oct. 24, 2008
click here to download today's notes in
more printer friendly Microsoft WORD format
A couple of songs from Pink Martini were featured before class
today. The first was "Let's never stop
falling in love," (no dancing in that video, if you want dancing
look at this video,
I'm not sure what was going on in this video). We
had time for a second song "Lilly."
Experiment #4 materials were
distributed in class today. There are a few remaining sets of
materials, I'll have them in class next Monday. It looks like
this will be a perfect weekend to gather Experiment
#3 data. Why not do so, then bring back your materials early
next week so that someone else can check them out.
The first part of the Quiz #3 Study Guide
is now available online. This study guide is a little more
interactive than previous study guides and includes a lot of example
problems (with answers).
We learned about sling psychrometers in class on
Wednesday. In class today I mentioned that I had added a little
more explanattion and discussion to the online notes (more information
than was covered in class on Wednesday). Here's an example:
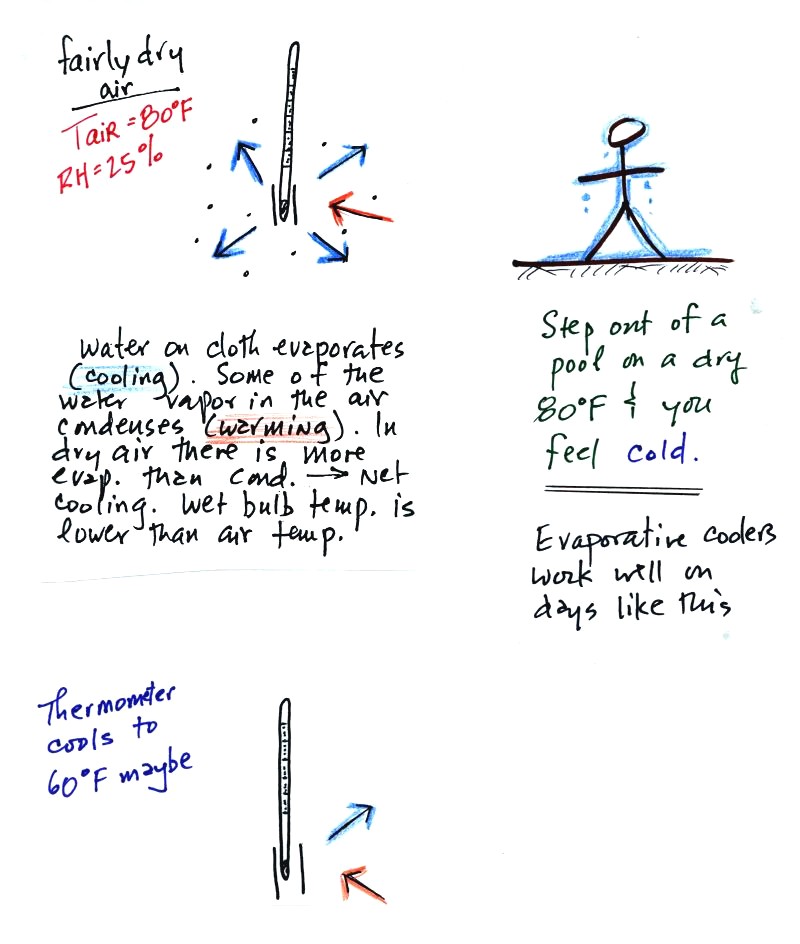
When you start to swing the psychrometer, water
will begin to evaporate from the wet piece of cloth. The amount
or rate of evaporation will depend on the water temperature (the
80 F
value was just made up in this example). Warm water evaporates at
a higher rate than cool water.
The evaporation is shown as blue arrows because this will cool the
thermometer. The same thing would happen if you were to step out
of a swimming pool on a warm dry day, you would feel cold. Swamp
(evaporative) coolers would work well on a day like this.
The figure at upper left also shows one arrow of condensation.
The amount or rate of condensation
depends on how much water vapor is
in the air surrounding the thermometer. In this case (low
relative humidity) there isn't much water vapor. The
condensation arrow is orange because the condensation will release
latent heat and warm the thermometer.
Because there is more evaporation (4 arrows) than condensation (1
arrow) the wet bulb thermometer will drop.
The wet thermometer will cool but it won't cool indefinitely. We
imagine that the wet bulb thermometer
has cooled to 60 F. Because the wet piece of cloth is cooler,
there is less evaporation. The wet bulb thermometer has cooled to
a temperature where the evaporation and condensation are in
balance. The thermometer won't cool any further.
You
would measure a large difference (20 F) between the dry and wet bulb
thermometers on a day like this when the air is relatively dry.
Here's something that wasn't mentioned in class last Wednesday.
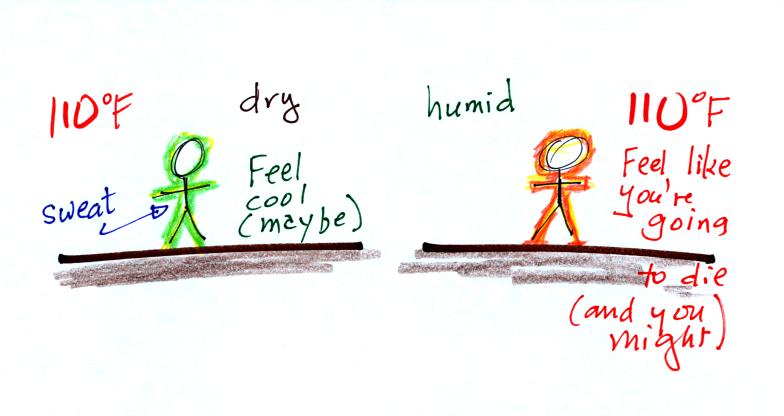
Our bodies try to keep themselves cool by perspiring
during
hot
weather. According to a textbook we used in a previous semester
"... over ten million sweat glands wet the
body with as much
as two liters of liquid/hour."
When the RH is
high, there might not be enough net evaporation to cool your
body.
You might end up with HEAT STROKE - a
potentially deadly
condition.
Wind and cold temperatures make it feel colder than it really
is,
High temperatures and high humidity make it feel
hotter than it is.
The wind chill temperature measures the effect of cold temperatures and
wind,
The heat index (p. 142 in the text) measures the effect of high
temperatures and high
relative humidities.
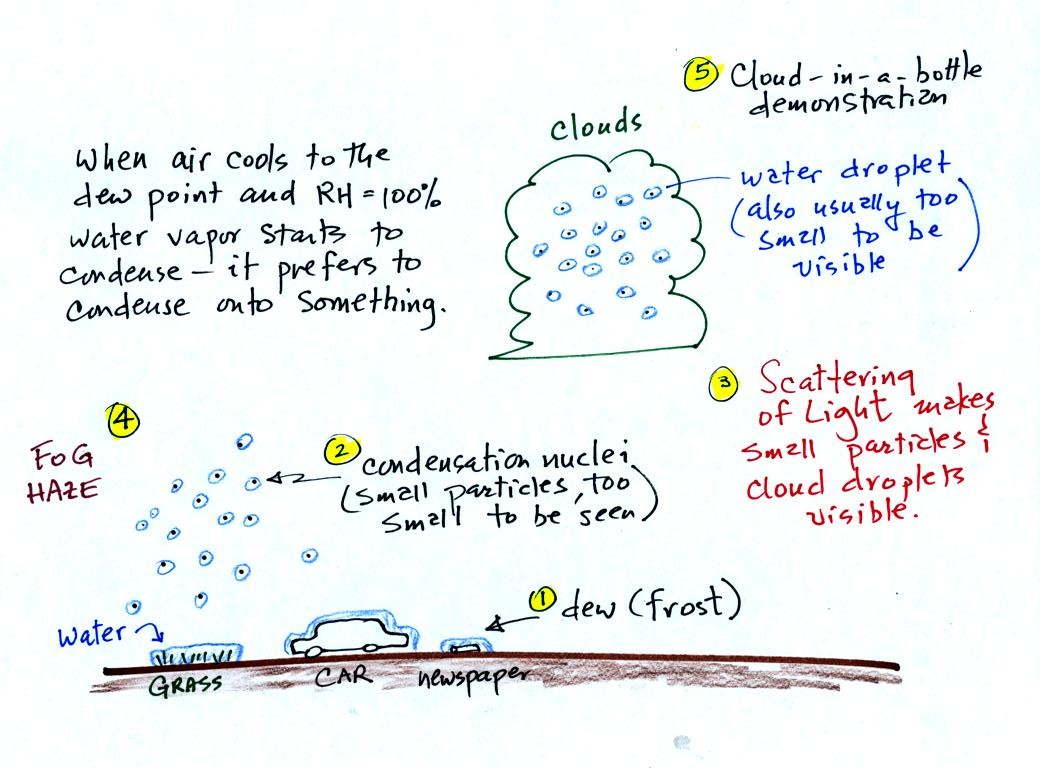
When moist air next to the ground is cooled to and
below the
dew point, water vapor condenses onto (or is deposited onto) the ground
or object on the ground. This is dew, frozen dew, and
frost. We covered this in class on Wednesday.
Air above the ground can also be cooled to the dew point. When
that happens it is much easier for water vapor to
condense
onto
something rather than just forming a small droplet of pure
water. In air above the
ground water vapor condenses onto small
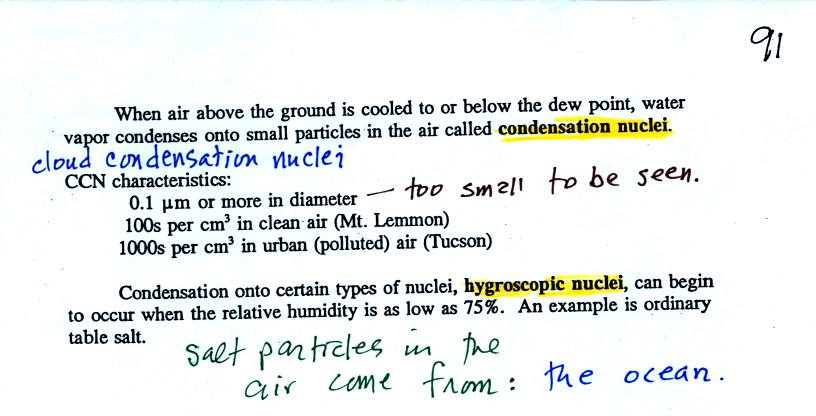
particles in the air called condensation nuclei.
You can learn why it is so hard to form small droplets of
pure water by
reading the top of p. 92 in the
photocopied class notes.
Water vapor will condense onto certain kinds of condensation
nuclei
even when the relative humidity is below 100% (again you will find some
explanation of this on the bottom of
p.
92). These are called hygroscopic
nuclei.
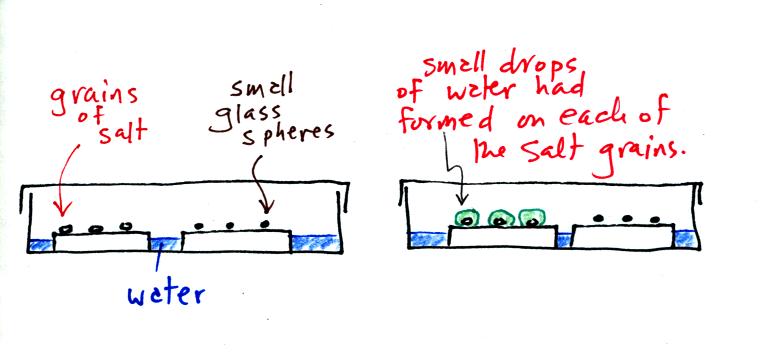
A short video showed how water vapor would, over time,
preferentially
condense onto small grains of salt rather than small spheres of
glass. The figure below
wasn't shown in class.
The start of the video at left showed the small grains
of
salt were
placed on a platform in a petri dish
containing water. Some small spheres of glass were placed in the
same
dish. After about 1 hour small drops of water had formed around
each
of the grains of salt (shown above at right).
In humid parts of the US, water will condense onto the grains of salt
in a salt shaker causing them to stick together. Grains of rice
apparently absorb moisture which keeps this from happening and allows
the salt to flow
freely out of the shaker when needed.
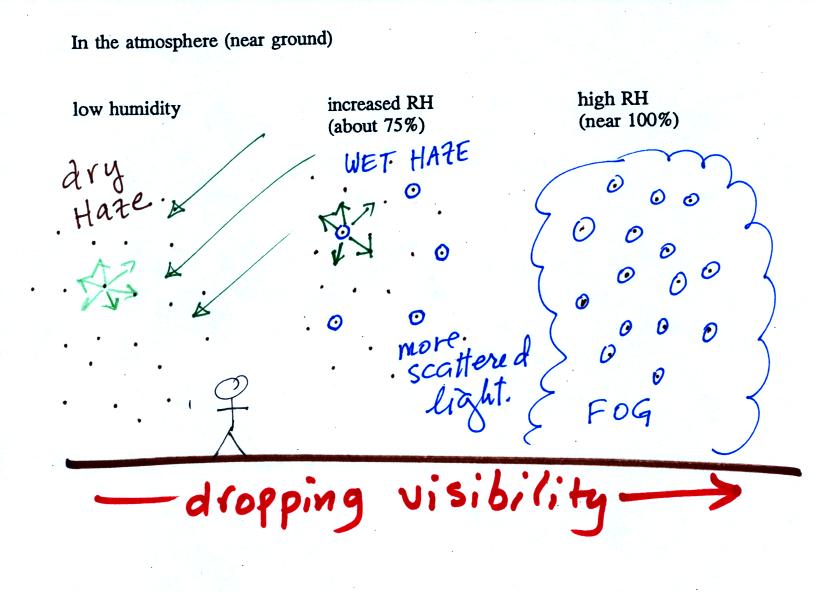
This figure (bottom of p. 91)
shows
how
cloud
condensation nuclei and increasing relative humidity can affect the
appearance of the sky and the visibility.
The air in the left most figure is relatively dry. Even
though
the condensation nuclei particles are too small to be seen with the
human eye you can tell they are there because they scatter
sunlight. When you look at the sky you see the deep blue color
caused by scattering of sunlight by air molecules mixed together with
some white
light scattered by the condensation nuclei. This changes
the color of the sky from a deep blue to a bluish white
color. The more particles there are the whiter the sky
becomes. This is called "dry haze."
The middle picture shows what happens when you drive from the dry
southwestern part of the US into the humid
southeastern US. One of the first things you would notice is the
hazier
appearance of the air and a decrease in visibility. Because the
relative humidity is high,
water vapor begins to condense onto some of the condensation nuclei
particles (the hygroscopic nuclei) in the air and forms small water
droplets. The water droplets scatter more sunlight than just
small particles alone. The increase in the amount of scattered
light is what gives the air its hazier appearance. This is called "wet
haze."
Finally when the relative humidity increases to 100% fog
forms.
Fog can cause a severe drop in the visibility. The thickest fog
forms in dirty air that contains lots of condensation nuclei. We
will see this effect in the cloud-in-a-bottle demonstration coming up
at the end of class.
There are two types of fog that you might occasionally see in
Tucson (fog is fairly infrequent because the air is so dry)
To produce fog you first need to increase the relative humidity (RH) to
100%
You can do this either by cooling the air or adding moisture to
and saturating the air (both will increase the ratio in the RH formula
above).
The ground cools during the night by emitting IR radiation (left figure
below). The ground cools most rapidly when the skies are free of
clouds and the air is dry (except for a thin layer next to the
ground). These are the conditions that favor the formation of
radiation fog.
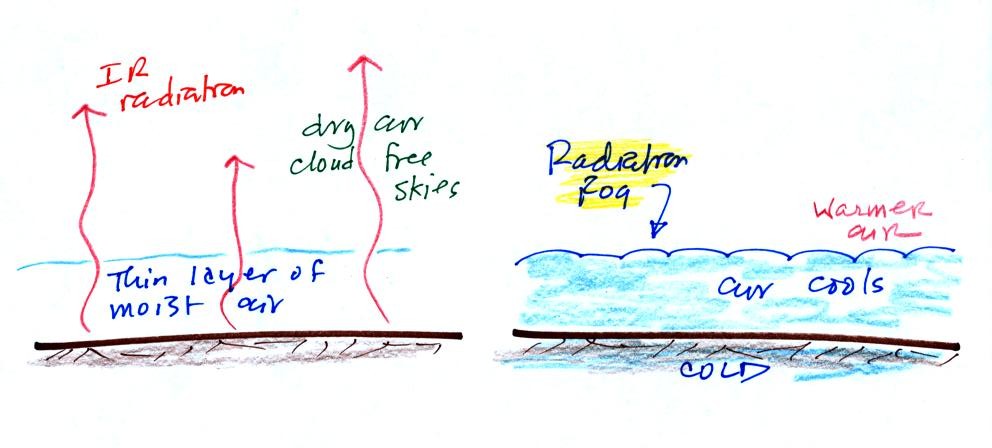
Air in contact with the ground cools and radiation fog can form (right
figure above). Because the fog cloud is colder than the air right
above, this is a stable situation. The fog clouds "hugs" the
ground.
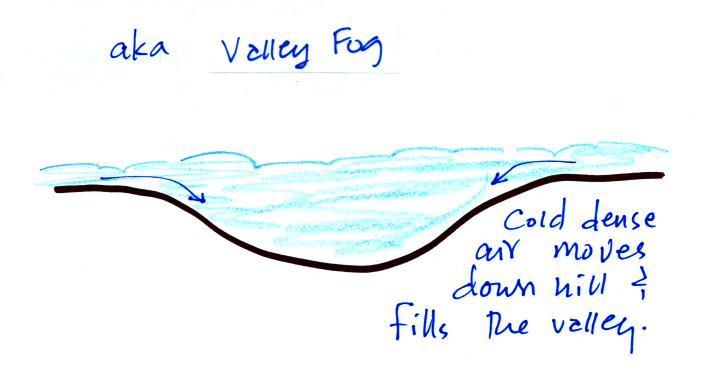
Radiation fog is sometimes called valley fog.
The cold dense air will move downhill and fill low lying areas with fog
cloud. It is often difficult for the sun to warm the air
and dissipate thick clouds of valley fog.
Steam fog (aka evaporation fog or mixing fog) is commonly observed on
cold mornings over the relatively warm water in a swimming pool.

Water evaporating from the pool
saturates the cold air above. Because the fog cloud is warmer
than the cold surrounding air, the fog clouds float upward.
When you "see your breath" on a cold day
you're seeing mixing fog. Warm moist air from your mouth mixes
with the colder air outside. The mixture is saturated and a fog
cloud forms.
It was time next for a cloud-in-a-bottle demonstration.
Cooling
air and
changing relative humidity, condensation nuclei, and scattering of
light are all involved in this demonstration.
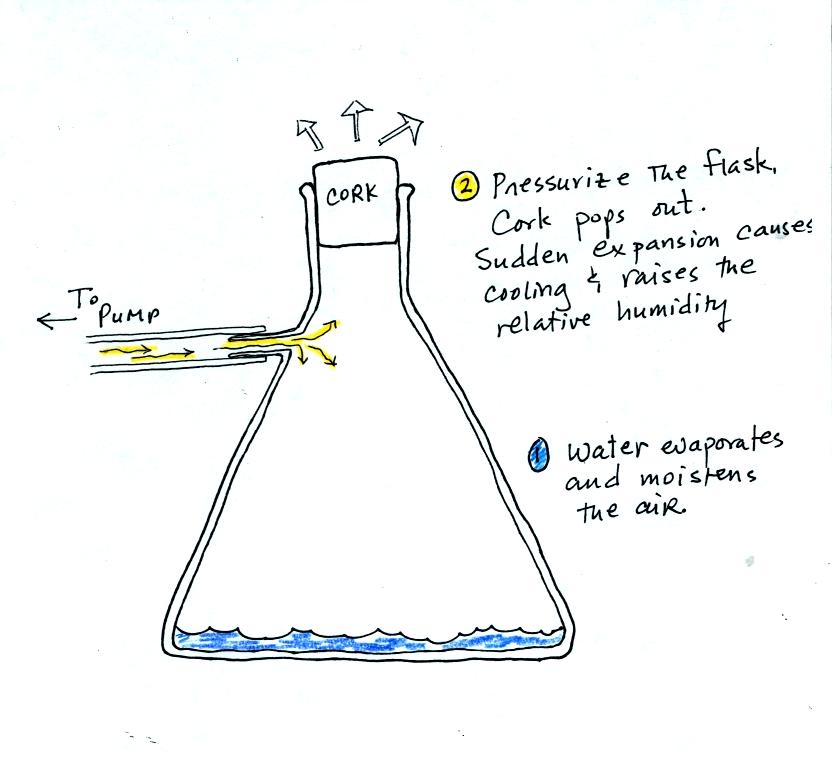
We used a strong thick-walled 4 liter flask (flasks
like this are designed to not implode when all of the air is pumped out
of them, they aren't designed to not explode when pressurized).
There
was a little
water in the bottom of the flask to moisten the air in the flask.
Next we pressurized the air in the flask. At some point the
pressure blows the cork out of the top of the flask.
The air in
the flask expands outward and cools. This sudden cooling
increases the
relative humidity of the moist air in the flask to 100% (probably more
than 100%) and water vapor condenses onto cloud condensation nuclei in
the air. A cloud became visible (barely) at this point. The
cloud droplets are too small to be seen with the human eye. You
can see the cloud because the water droplets scatter light.
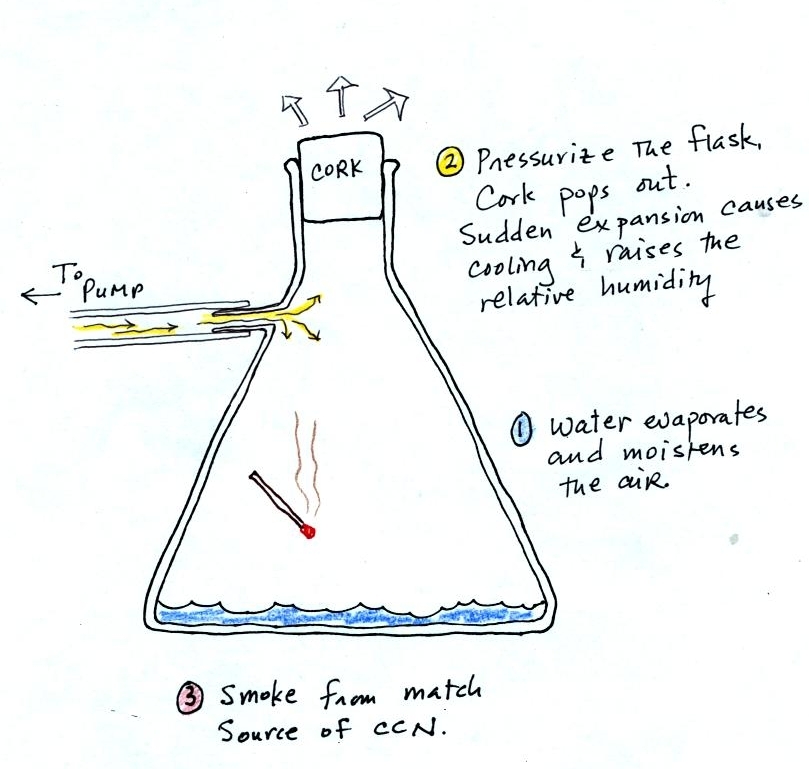
The demonstration was repeated an
additional time with one
small
change. A burning match was dropped into the
bottle. The smoke from the match added lots of very small
particles, condensation nuclei, to the air in the flask. The
cloud that formed
this time was quite a bit "thicker" and much easier to see.
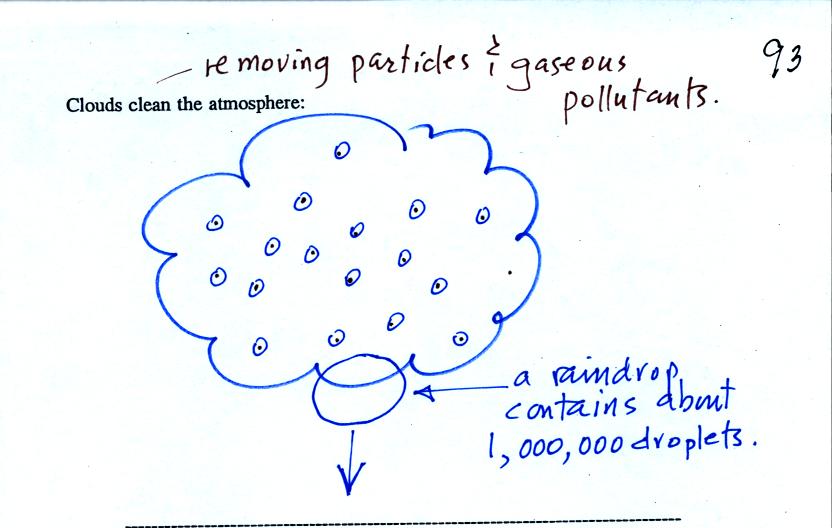
Clouds are one of the best ways of cleaning the
atmosphere
(cloud
droplets form on particles, the droplets clump together to form a
raindrop, and the raindrop carries the particles to the ground).
A raindrop can contain 1 million cloud droplets so a single raindrop
can remove a lot of particles from the air. You may have noticed
how clear the air seems the day after a rainstorm. Gaseous
pollutants can dissolve in the water droplets and be carried to
the ground by rainfall also.

A cloud that forms in dirty air is composed of a large
number of small droplets (right figure above). This cloud is more
reflective
than a cloud that forms in clean air, that is composed of a smaller
number of larger
droplets (left figure).
Just like in the cloud-in-a-bottle demonstration, the cloud that was
created when the air was full of smoke particles was much more visible
than the cloud made with cleaner air.
This is has implications for climate change.
Combustion of fossil fuels adds carbon dioxide to the atmosphere.
There is concern that increasing carbon dioxide concentrations will
enhance the greenhouse effect and cause global warming.
Combustion also adds condensation nuclei to the atmosphere (just like
the burning match added smoke to the air in the flask). More
condensation nuclei might make it easier for clouds to form, might make
the clouds more reflective, and might cause cooling. There is
still quite a bit of uncertainty how clouds might change and how this
might affect climate (remember too that clouds are good absorbers of IR
radiation).