Friday Sept. 12, 2008
Click here for a more printer friendly version
of these notes in Microsoft WORD format.
"Born to be a Bachelor" by Gaelic
Storm was the song played before class today.
For the latest news on Hurricane Ike as in makes landfall you might
check the website for the Houston
Chronicle newspaper.
The Practice Quiz has been graded and was returned in class
today. The 64% average is pretty typical for a practice
quiz. The average on Quiz #1 will most likely be higher, but not
necessarily a lot higher. You'll find answers to all the Practice
Quiz questions here.
An In-class Optional
Assignment was handed out in class today, it was collected at the
end of the period. If you download the assignment, answer the
questions, and turn in the assignment at the beginning of class on
Monday you can earn partial credit.
We reviewed the concepts of mass, weight, and density before the
quiz last Wednesday. Today we will learn about pressure.
The air
that
surrounds the earth has mass. Gravity pulls downward on the
atmosphere giving it weight. Galileo conducted (in the 1600s) a
simple
experiment to prove that air has weight.
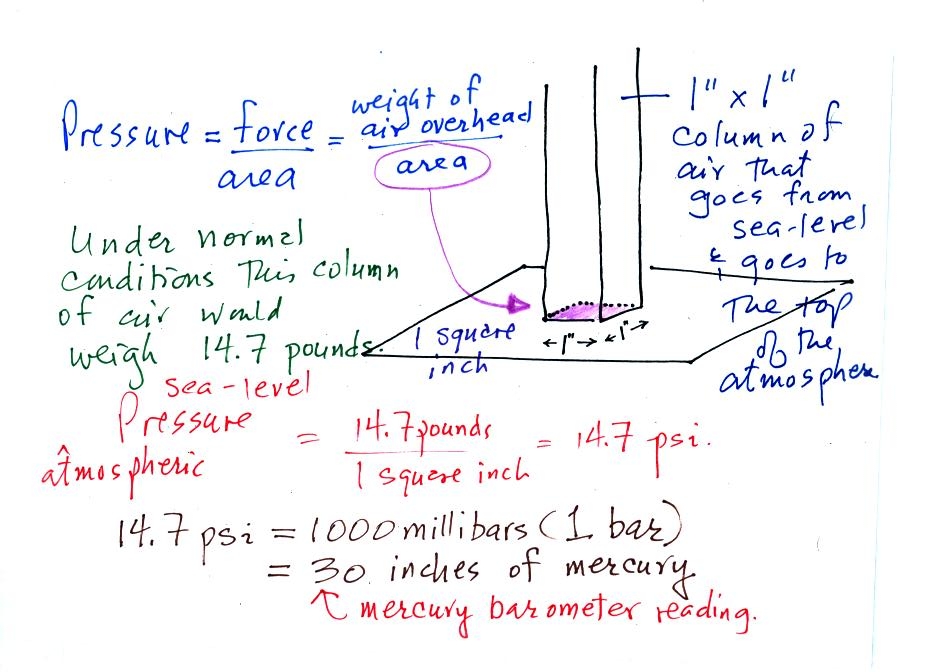
Pressure is defined as force divided by area. Air
pressure is the
weight
of the atmosphere overhead divided by the area the air is resting
on.
Atmospheric pressure is
determined by and tells you something about the weight of the air
overhead.
Under normal conditions a 1 inch by 1 inch column of air stretching
from sea level to the top of the atmosphere will weigh 14.7
pounds. Normal
atmospheric
pressure at sea level
is 14.7 pounds per square inch (psi, the units you use when you fill up
your car or bike tires with air).
The iron bar that was passed around class on Wednesday also
weighed 14.7 pounds. The
following figure wasn't shown in class.
Steel is a lot denser than air, so the steel bar only needed to be
52 inches tall. The air column is a 100 miles or more tall.
Some of the other commonly used pressure units are shown
above and repeated below.
Typical sea level pressure is 14.7 psi or about 1000 millibars
(the
units used by meterologists and the units that we will use in this
class most of the time) or about 30 inches of mercury (refers to
the reading on a mercury barometer). If you ever find
yourself in France needing to fill your
automobile tires with air (as I once was, I owned a Peugeot 404)
remember that the air compressor scale is
probably calibrated in bars. 2 bars of pressure would be
equivalent to 30 psi.
The word "bar" is used in a lot of meteorological terms:
Pressure
at sea level is determined by the weight of the air overhead.
What about pressure at some level above sea level?
We can use a stack of bricks to try to answer this
question.
Each brick
weighs 5 pounds. At the bottom of the 5 brick tall pile you would
measure a weight of 25 pounds. If you moved up a brick you would
measure a weight of 20 pounds, the weight of the four bricks still
above. In the atmosphere, pressure at any level is determined by
the weight of the air still overhead. Pressure decreases with
increasing altitude because there is less and less air remaining
overhead. The numbered points on the figure below were added
after class.
At sea
level altitude, at Point 1,
the pressure is normally about 1000 mb. That is determined by the
weight of all (100%) of the air in the atmosphere.
Some parts of Tucson, at Point 2, are 3000
feet above sea level (most
of the valley is lower than that). At 3000 ft. about 10% of the
air is
below, 90% is still overhead. It is the weight of the 90% that is
still above that determines the atmospheric pressure in Tucson.
If 100% of the atmosphere produces a pressure of 1000 mb, then 90% will
produce a pressure of 900 mb.
Pressure is typically about 700 mb at the
summit of Mt. Lemmon (9000
ft. altitude at Point 3) and 70% of the atmosphere is overhead..
Pressure decreases rapidly with increasing
altitude. We will find that pressure changes more slowly if you
move horizontally. It is small horizontal changes that cause the
wind to blow however.
Point 4 shows a submarine at a depth of
about 33 ft. The pressure
there is determined by the weight of the air and the weight of the
water overhead. Water is much denser and much heavier than
air. At 33 ft., the pressure is already twice what it would be at
the surface of the ocean.
The person in the picture below (from a Physics textbook) is 20 deep
underwater. There is a large pressure pushing against his body
from the surrounding water. If he puts his mouth on the snorkel
(which runs up to the top of the water) he will collapse his
lungs. The snorkel is connected to atmospheric pressure which is
quite a bit lower than the pressure at 20 depth in the water.
Next
we'll learn that the rate of pressure decrease with increasing altitude
depends on the air's density. I borrowed the figure below from
the other section of the class because this picture is a little clearer
than the one we produced on Friday.
There is a lot going on in this picture. 1000 mb at Point
1 is a typical value for sea level pressure. The fact that
the
pressures are equal at the bottoms of both
sides of the picture means that the weight of the atmosphere at the
bottom of the
picture on the left is the same as the weight of the atmosphere at the
bottom of the picture at right. The only way this can be true is
if there is the same total amount (mass) of air in both cases.
Point 2 - Moving upward from the ground we find that pressure decreases
to 900 mb at the level of the dotted line in the picture at left.
This is what you expect, pressure decreases with increasing
altitude. In the figure at right you need to go a little bit
higher for the same 100 mb decrease.
Since there is a 100 mb drop in both the layer at left and
in
the
layer at right, both layers must contain the same amount (mass) of air.
Point 3 - The most rapid rate of pressure decrease with increasing
altitude is occurring in the picture at left.
Point 4 - The air in the picture at left is squeezed into a thinner
layer than in the picture at right. The air density in the left
layer is higher than in the layer at right.
By carefully analyzing this figure we have proved to ourselves that the
rate of pressure decrease
with altitude is higher in dense air than in lower
density air.
This is a fairly subtle but important concept. We will use this
concept several times during the semester. In particular we will
need this concept to understand why hurricanes can intensify and get as
strong as they do.
Newton's
Law of Universal Gravitation is an equation that allows you to
calculate the gravitational attraction between two objects. We
really
didn't
work through the following figures in class (except for filling
in the
boxes at the bottom of p. 28 with the English and Metric units for mass
and weight). The reason that they have been included on the
online notes is that with a little thought you can appreciate and
understand why certain variables appear in Newton's Law and why they
appear in either the numerator (direct proportionality) or in the
denominator (inverse proportionality).

The gravitational attraction
between two
objects (M and m in
the figures) depends
first of
all
on the distance separating the objects. The gravitational
force becomes weaker the further away the two objects are from each
other. In the bottom
picture above and the top figure below we see that the attractive force
also depends on the masses of the two objects.

The complete formula is shown in
the middle of the page
above. G
is a constant. On the surface of the earth G, M, and R don't
change. The gravitational acceleration, g, is just the
quantity [G times Mearth
divided by ( Rearth )2 ]. To determine the
weight (on the earth's surface) of an object
with mass m you simply multiply m x g.
Down at the bottom of the page are the Metric and English units of
mass
and weight. You have probably heard of pounds, grams, and
kilograms. You might not have heard of dynes and Newtons.
Most people have never heard of slugs.
Here's another page from the
photocopied Class Notes that we didn't cover in class. The
weight of a person on the earth and the moon is calculated in English
and metric units.
The mass of a person would be the same on the earth and on the moon.
The weight of a person depends on the person's mass and on the strength
of gravity (the acceleration of gravity term, the g variable below).
(1) After a long cold winter and
without much bicycling or other exercise, the course instructor
sometimes weighs as much as 160 pounds. In
(2) we see that the
gravitational acceleration (g) is 32 ft/sec2 in English units (on the
earth). The
meaning of this value is shown in (3). If you drop an object it
will start to fall and will speed up as it continues to fall.
Gravity will cause a
falling object to fall 32 ft/sec faster every second. Dividing
the instructor's weight by the gravitation
acceleration in (4) we obtain the instructor's mass, 5 slugs, in
English units.
In metric units, the instructor has a mass of 73 kilograms
(5).
The gravitation acceleration in metric units is 9.8 m/sec (6).
Multiplying these
two values, in (7), we find that the instructor weighs 715 Newtons.
On the moon, the mass stays the same. Gravity is weaker, so
the
value of g is smaller. The instructor would weigh quite a bit
less (117 Newtons or 26 pounds) on the moon compared to the earth.
Mercury
barometers are used to measure atmospheric pressure. A mercury
barometer is really just a balance that can be used to weigh the
atmosphere. A basic understanding of how a mercury barometer
works is something that every college graduate should have.
You'll find most of what follows on p. 29 in the
photocopied Class Notes.
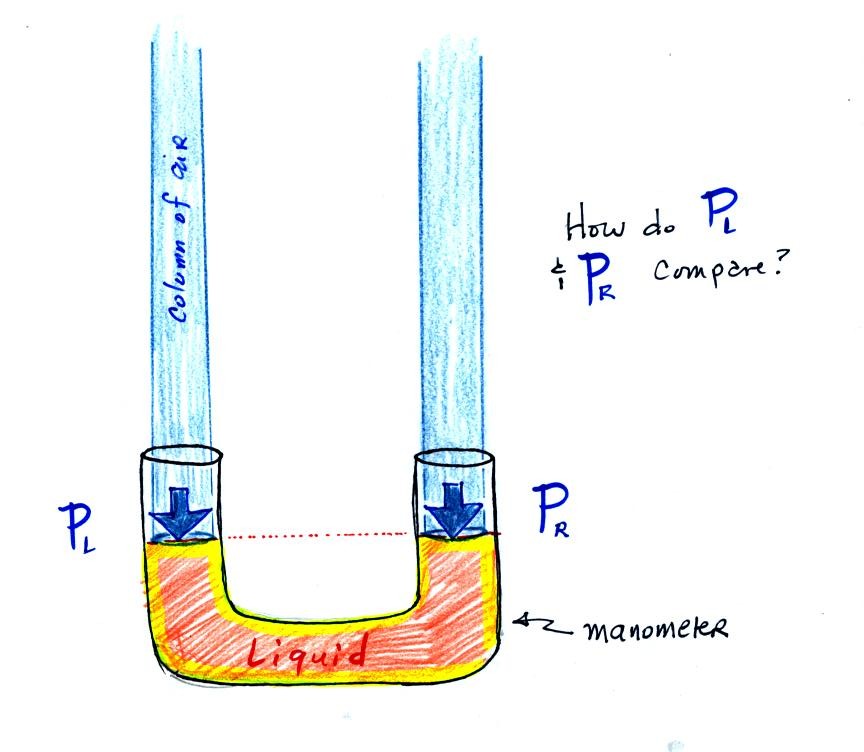
The instrument above ( a u-shaped
glass
tube filled with a
liquid of some kind) is a manometer and can be used to measure pressure
difference. The
two ends of the tube are open so that air can get inside and air
pressure can press on the liquid. Given that the liquid levels on
the two sides of the manometer
are equal, what could you about PL and PR?
The liquid can slosh back and
forth just like the pans on a balance can move up and down. A
manometer really behaves just like a pan balance.
PL and PR
are equal (note
you don't really know what either pressure is just that they are equal).
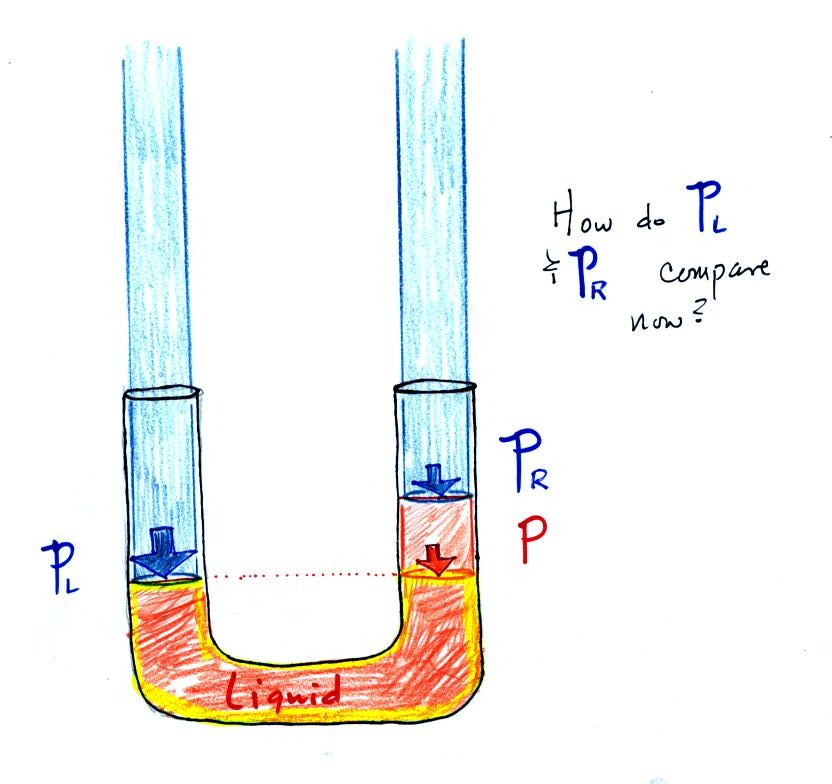
Now the situation is a little
different,
the
liquid levels
are no
longer equal. You probably realize that the air pressure on the
left, PL, is a little higher than the air pressure on the
right,
PR. PL is now being balanced by PR
+ P acting together. P
is the pressure produced by the weight of the extra fluid on the right
hand side of
the manometer (the fluid that lies above the dotted line). The
height of the column of extra
liquid provides a measure of the difference between PL and PR.
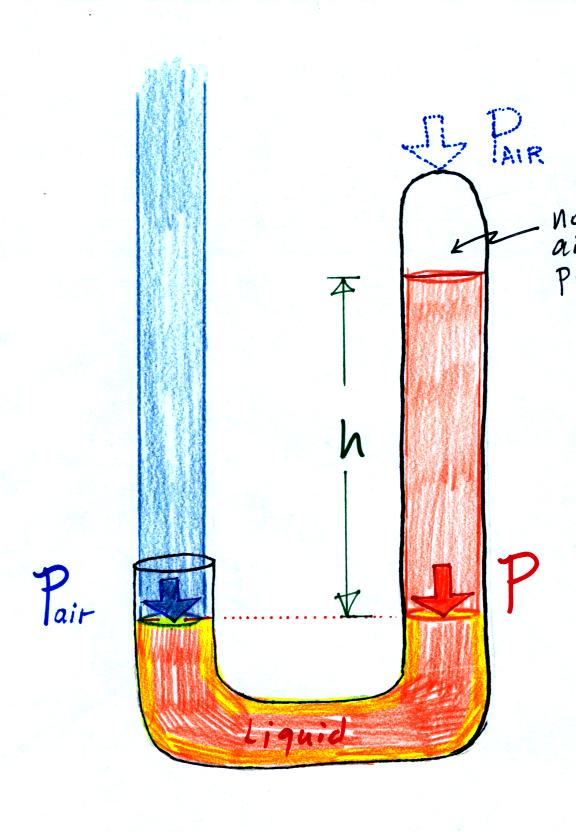
Next we will go an extreme and close off the right hand side of the
manometer.
Air pressure can't get into the
right tube any
more. Now at the level of the dotted line the balance is between
Pair and P (pressure by the extra liquid on the
right). If
Pair
changes, the height of the right column, h, will
change. You now have a barometer, an instrument that can measure
and monitor the atmospheric pressure. (some of the letters were cut off
in the upper right portion of the figure, they should read "no air
pressure")

Barometers like this are usually
filled with mercury. Mercury is
a liquid. You need a liquid that can slosh back and forth in
response to changes in air pressure. Mercury is also dense which
means the barometer won't need to be as tall as if you used something
like water. A water barometer would need to be over 30 feet
tall. With mercury you will need only a 30 inch tall column to
balance the weight of the atmosphere at sea level under normal
conditions (remember the 30 inches of mercury pressure units mentioned
earlier). Mercury also has a low rate of
evaporation so you don't have much mercury gas at the top of the right
tube.
Finally here is a more conventional
barometer design.
The bowl of
mercury is usually covered in such a way that it can sense changes in
pressure but not evaporate and fill the room with poisonous mercury
vapor.