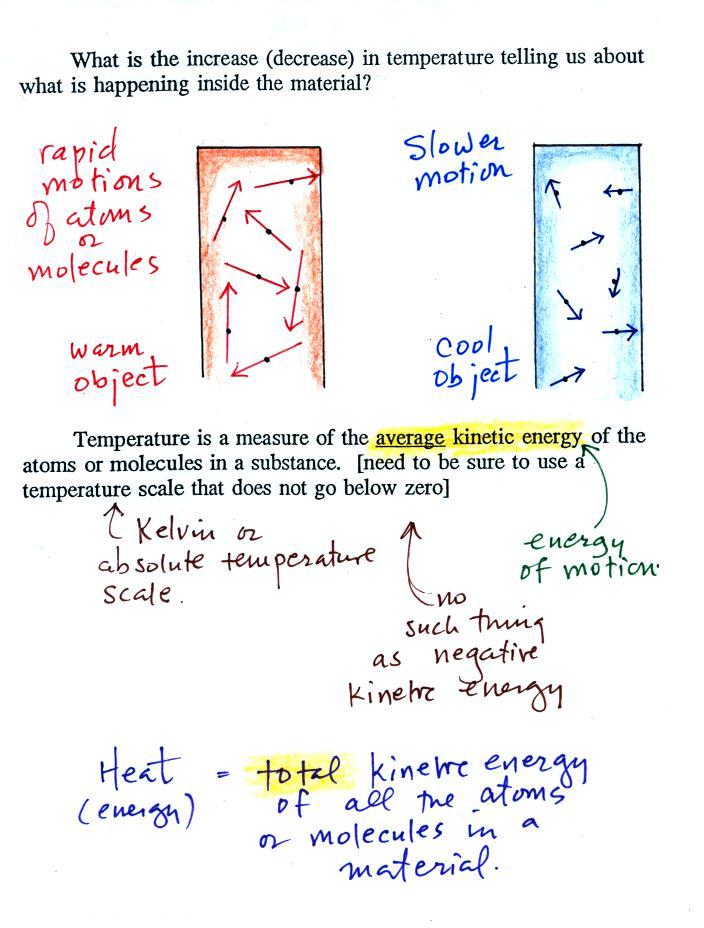
The atoms or molecules inside
the warmer object will be moving more rapidly (they'll be
moving freely in a gas, just "jiggling" around while still
bonded to each other in a solid). Temperature provides a
measure of the average
kinetic energy of the atoms or molecules in a material.
You need to be careful what temperature scale you use when
using temperature as a measure of average kinetic
energy. You must use the Kelvin temperature scale
because it does not go below zero (0 K is known as absolute
zero). The smallest kinetic energy you can have is zero
kinetic energy. There is no such thing as negative
kinetic energy.
You can think of heat as being the total kinetic
energy of all the molecules or atoms in a material.
This next figure might make
clearer the difference between temperature (average kinetic
energy) and heat (total kinetic energy). This
figure wasn't shown in class.
A cup of water and a pool of
water both have the same temperature. The average
kinetic energy of the water molecules in the pool and in the
cup are the same. There are a lot more molecules in
the pool than in the cup. So if you add together all
the kinetic energies of all the molecules in the pool you
are going to get a much bigger number than if you sum the
kinetic energies of the molecules in the cup. There is
a lot more stored energy in the pool than in the cup.
It would be a lot harder to change the total energy of the
water in the pool, i.e. cool (or warm) all the water in the
pool, than it would be to change the total energy of the
water in the cup.
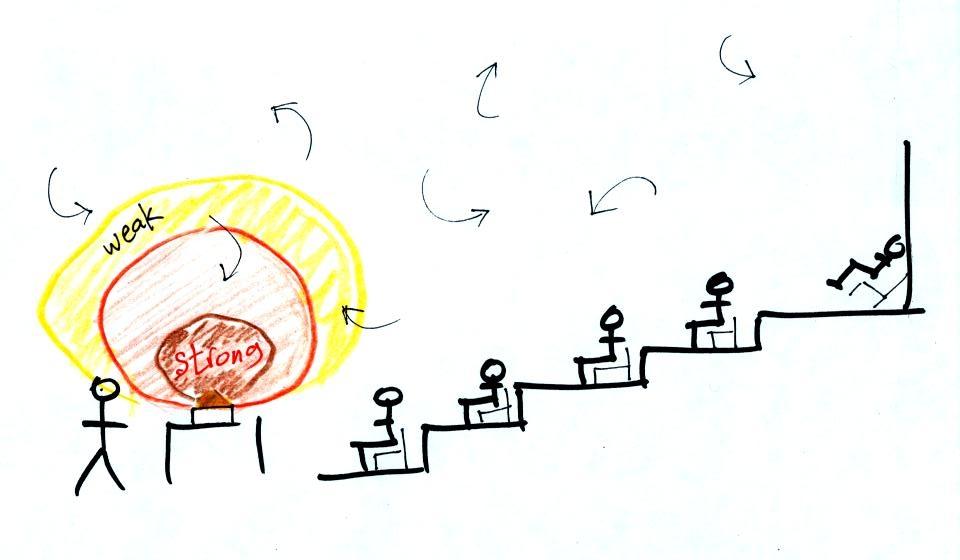
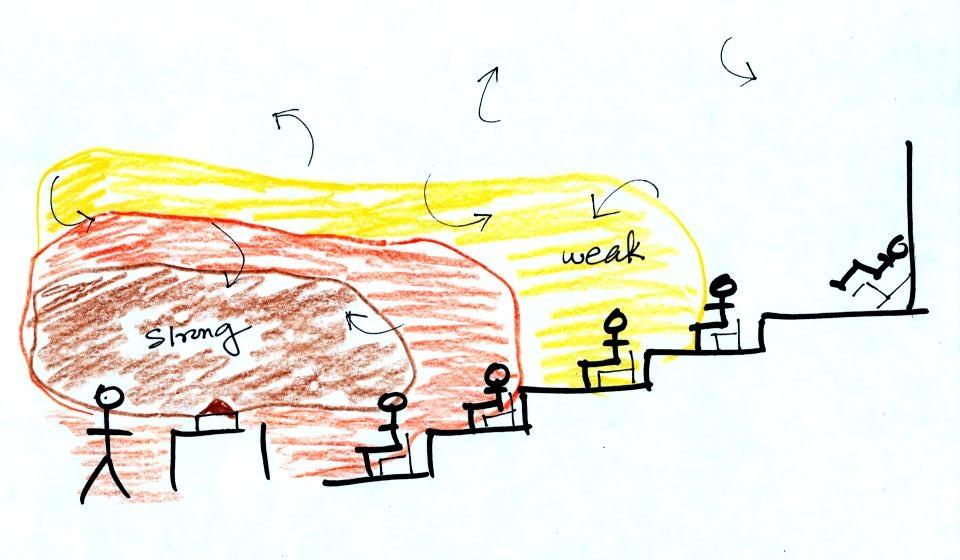
The difference between
temperature and heat can be understood by considering groups
of people and money (the people represent atoms or molecules
and the money is analogous to kinetic energy). Both
groups above have the same $10 average amount of money per
person (that's analogous to temperature). The $100
held by the larger group at the left is greater than the $20
total possessed by the smaller group of people on the right
(total amount of money is analogous to heat).
And finally, speaking of
temperature scales

You should remember the
temperatures of the boiling point and freezing point of
water on at least the Fahrenheit and Celsius scales
(and the Kelvin scale if you want to). 300 K is a
good easy-to-remember value for the global annual average
surface temperature of the earth. Remember that
number and also that temperature never goes below zero on
the Kelvin scale.
And on to the in-class
experiment. A couple of students from the
class were nice enough to volunteer to perform the experiment
(they were given green cards as compensation).
Here's the object of the experiment:
The students that are doing Experiment #2 are doing
something similar, they are measuring the latent heat of
fusion of ice, the energy needed to melt one gram of
ice.
Here's the data that the
students collected as best I can tell (I gave my notes to a
student after class). This would be hard to figure out
even after having cleaned things up a bit after class.
So here's a step by step explanation of what the students
did:
(a)
Some room temperature water poured into a styrofoam cup
weighed 159.6 g. The cup itself weighed 3.9 g, so they had
155.7 g of water. The water's temperature was
measured with the thermometer and was 20.0 C (room temperature).
(b)
Some liquid nitrogen was poured into a second smaller
styrofoam cup. That weighed 49.0 g. Subtracting the
2.0 g weight of the cup means we had 47.0 g of liquid
nitrogen.
We don't need to measure the temperature of the liquid
nitrogen (doing so would probably destroy the
thermometer). It had already warmed up as much as it could
( to -320 F as mentioned earlier). Any additional energy
added to the liquid nitrogen will cause it to evaporate.
(c)
After the liquid nitrogen had evaporated the water's
temperature was remeasured. It had dropped to 4.5 C.
We started out with water that
was 20.0 C, so that is a temperature drop of 15.5 C.
It takes energy to turn liquid nitrogen into nitrogen
gas. The energy needed will be taken from the water (the
red arrow below, energy naturally flows from hot to
cold).
Because the experiment was
performed in an insulated styrofoam cup we will assume all of
the energy taken from the water is used to evaporate
nitrogen. Minimal energy flows into the room air or
anything like that. We will set the two equations above
equal to each other. This is an energy balance equation,
energy taken from the room temperature water at left and
energy needed to evaporate the liquid nitrogen at right.
We know the mass of the
nitrogen that we started with and that was eventually
evaporated (47.0 g) and the mass of the water (155.7 g).
We measured the ΔT (15.5 C) and we know
the specific heat of water (1 cal/g C). We substitute
them into the equation above and solve for LH, the latent heat
of vaporization of liquid nitrogen. Here are the details
of the calculation:
A responsible &
trustworthy student in the class informed us that the known
value is 48 cal/g, so this measured value is pretty close to
the known value. We measured 51.9 cal/g and
52.7 cal/g in my two classes last fall.
Conduction is the first of four energy transport processes
that we will cover (and the least important transport process in
the atmosphere). The figure below illustrates this
process. Imagine heating the end of a piece of copper
tubing just so you ccan visualize a hot object. If you
held the object in air it would slowly lose energy by conduction
and cool off.
How does that happen? In the top picture some of the
atoms or molecules near the hot object have collided with the
object and picked up energy from the object. This is
reflected by the increased speed of motion or increased kinetic
energy of these molecules or atoms (these guys are colored
orange).
In the middle picture the initial bunch of energetic
molecules have collided with some of their neighbors and shared
energy with them (these are pink). The neighbor molecules
have gained energy though they don't have as much energy as the
molecules next to the hot object.
In the third picture molecules further out (yellow) have now
gained some energy. The random motions and collisions
between molecules is carrying energy from the hot object out
into the colder surrounding air.
Conduction transports energy from hot to cold. The
rate
of
energy
transport
depends
first
on
the
temperature
gradient
or
temperature
difference
between
the
hot object and the cooler surroundings. If the object in
the picture had been warm rather than hot, less energy would
flow and energy would flow at a slower into the surrounding air.
The rate of energy transport also depends on the material
transporting energy (air in the example above). Thermal
conductivities of some common materials are listed. Air is
a very poor conductor of energy and is generally regarded as an
insulator.
Water is a little bit better conductor. Metals are
generally very good conductors (cooking pans are often made of
stainless steel but have aluminum or copper bottoms to evenly
spread out heat when placed on a stove). Diamond has a
very high thermal conductivity (apparently the highest of all
known solids). Diamonds are sometimes called "ice."
They feel cold when you touch them. The cold feeling is
due to the fact that they conduct energy very quickly away from
your warm fingers when you touch them.
I usually bring a propane torch to class to demonstrate the
behavior of materials with different thermal
conductivities. But I was carrying too much other
stuff. I'll bring the torch on Wednesday to demonstrate
conduction and also convection.
A piece of copper tubing is held in the flame in the picture
at left. Copper is a good conductor. Energy is
transported from the flame by the copper and you must grab the
tubing several inches from the end to keep from burning your
fingers. Part of a glass graduated cylinder is held in the
flame in the center picture. You could comfortably hold
onto the cylinder just a couple of inches from the end because
glass is a relatively poor conductor. The end of the glass
tubing got so hot that it began to glow (its is emitting radiant
energy, the 4th of the energy transport processes we will
discuss). Air is such a poor conductor that it is safe to
hold your finger just half an inch from the hot flame and still
not feel any heat coming from the flame.
Transport of energy by conduction is similar to the transport
of a strong smell throughout a classroom by diffusion.
Small eddies of wind in the classroom blow in random directions
and move smells throughout the room. For a demonstration
you need something that has a strong smell but is safe to
breathe.
I chose curry powder.
With time I was hoping the smell would spread throughout the
room. But ILC 130 is too large and the ventilation system
is too good. It quickly replaces air in the classroom with
fresh air from outside (if mercury were ever spilled I'm
guessing the ventilation system won't allow the vapor to build
up the dangerous levels). On Wednesday we'll add another
element to this demonstration and try to show why convection
(the 2nd energy transport we will study) is a more important
energy transport process than conduction.
Because air has such a low thermal conductivity it is often
used as an insulator. It is important, however, to keep
the air trapped in small pockets or small volumes so that it
isn't able to move and transport energy by convection (we'll
look at convection shortly). Here are some examples of
insulators that use air:
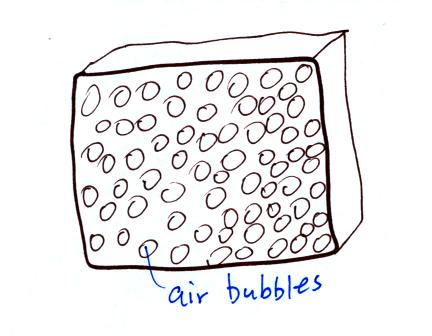
Foam is often used as an
insulator. Foam is filled with lots of small air
bubbles, they're what provides the insulation.
Thin insulating layer of air in a double pane
window. I don't have double pane
windows in my house. As a matter of fact I leave a
window open so my cats can get in and out of the house
(that's not particularly energy efficient).
You might need your winter coat on Wednesday it's
going to get cold.
Hollow fibers (Hollofil) filled with air used in
sleeping bags and ski coats.
Goose feathers (goosedown) work in a similar way.
Fiberglas insulation is another example. It works so
well as an insulator first because it is glass which has low
thermal conductivity and also because it traps lots of
little pockets of air.
We were about out of time at this point. Lots more to
come on Wednesday. You'll be surprised at how often
energy transport shows up in our daily lives.